61 Respiratory Levels of Organization
Our bodies exchange oxygen for carbon dioxide at a number of different levels. The exchange of air from the outside environment into the lungs is driven by the mechanics of ventilation. At a molecular level oxygen binds to hemoglobin in the red blood cells in the capillaries of the lungs. Some of this oxygen displaces carbon dioxide that was transported from peripheral cells. The exchange of gases occurs in red blood cells (where hemoglobin is concentrated) at the interface of the circulatory system and respiratory system, called the respiratory membrane. Oxygen diffuses from the inhaled air in the lungs across the aveolar and capillary membranes and into the blood plasma. It then enters the red blood cells where it will be carried on hemoglobin molecules to the other tissues of the body. Gas exchange at the respiratory membrane is known as external respiration. Gas exchange at between the tissues and the blood is internal respiration. At the molecular level carbon dioxide created during cell metabolism diffuses across the cell membrane into the interstitial spaces and extracellular fluid. When it crosses the capillary and red blood cell membrane some carbon dioxide is picked up hemoglobin by molecules inside the red blood cell. Inside the cells of the body tissues, oxygen will be used during the process of aerobic cellular respiration. Cellular respiration is a process used by our cells to convert nutrients into energy. The process of aerobic cellular respiration requires oxygen and produces carbon dioxide as a waste product.
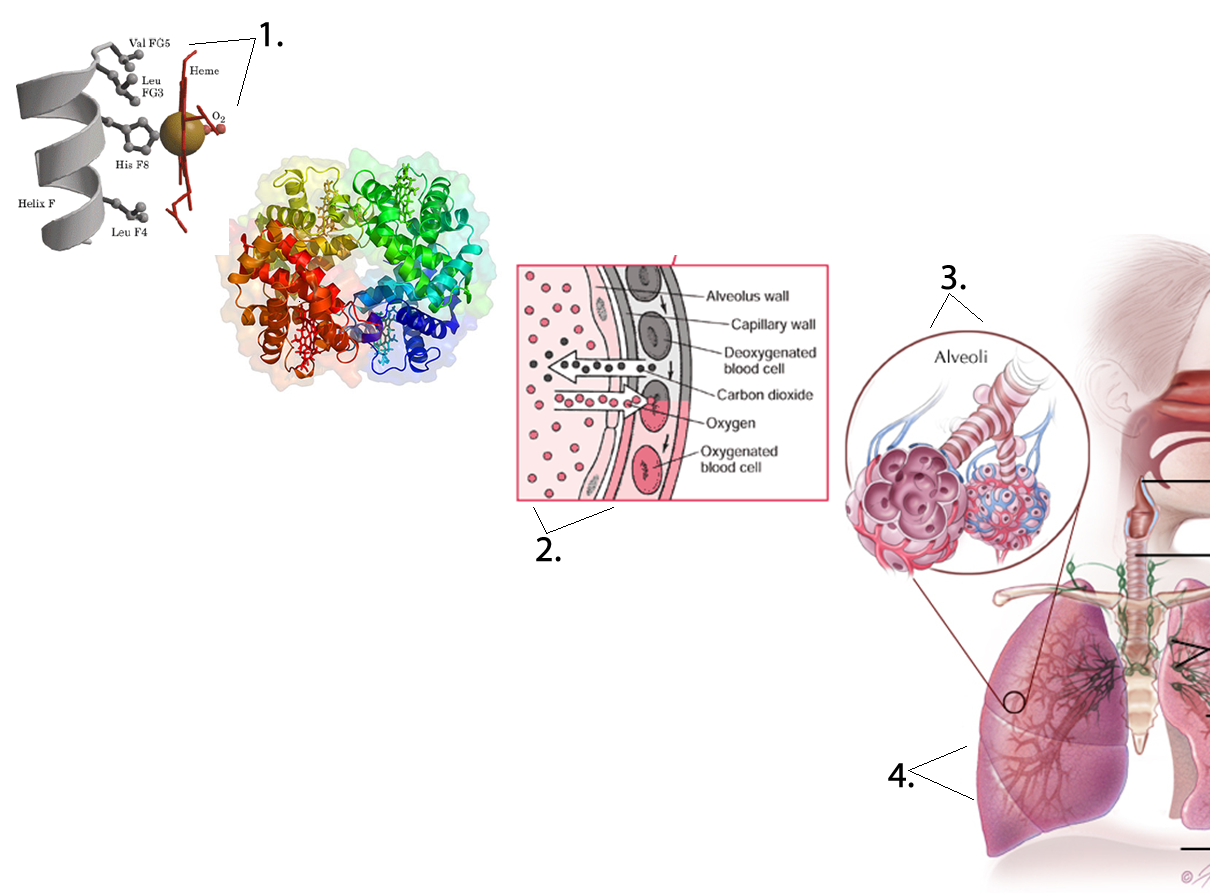
To understand these system-level functions, you will be further exploring structures and processes occurring at all levels of organization. Some examples of major respiratory structures assigned to their structural level of organization of the respiratory system include:
- Chemical level – oxygen, carbon dioxide, bicarbonate and hydrogen ions
- Macromolecular level – hemoglobin, mucus, and surfactant
- Cellular level – ciliated cells, goblet cells, alveolar cells, and macrophages
- Tissue level – stratified to pseudostratified to simple squamous epithelium
- Organ level – upper respiratory tract, bronchial tree and lungs
- Organ system level – integration of organs for gas exchange
Chemicals and Macromolecules
How Gases Exchange
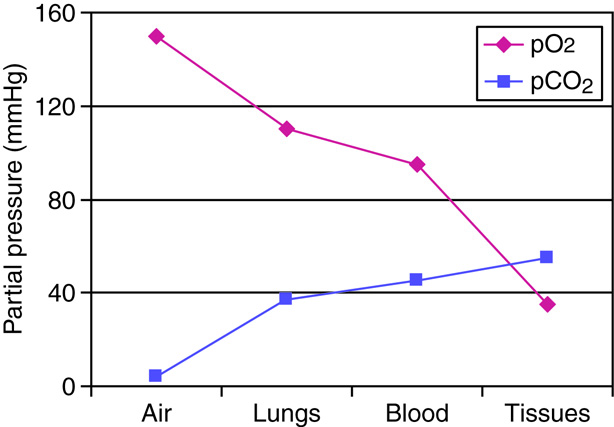
The four main gases in the atmosphere are nitrogen at 78.084%, oxygen at 20.946%, argon at 0.934%, and carbon dioxide at 0.039%. Other elements that account for less than 0.002% of the whole including neon, helium, krypton, hydrogen, nitrous oxide, carbon monoxide, xenon, ozone, nitrogen oxide, and trace amounts of ammonia. Water vapor ranges from 1 to 4 percent at sea level and 0.4% overall. The partial pressure of each of these elements can be found by multiplying the percentage of the particular gas by 1 atm or 760 mmHg, so the partial pressure of oxygen (PO2) is 760 x 0.20946, or 159.19 mmHg. The PCO2 is 760 x 0.0039, or 3 mmHg.
The respiratory system brings in oxygen and exchanges it for carbon dioxide. Oxygen makes up 21% of the air we breathe while carbon dioxide is found at very low levels (0.039%). The diffusion of gases across membranes follows the same principles as the diffusion of solutes in solution across membranes, basically molecules move down a gradient. The difference is that gases in solution are measured in terms of partial pressure.
Partial pressure is the pressure that a given gas in a mixture contributes to the total pressure inside the container or in the atmosphere. The partial pressure is equal to the total pressure times the fraction of the gas.
For example, at sea level, total atmospheric pressure is 760 mmHg. Because 21% of air is oxygen, the partial pressure of oxygen in atmospheric air at sea level is 760 mmHg x 21% = 160 mmHg. At higher altitudes, where atmospheric pressure may only be 500 mmHg, the partial pressure of oxygen would be 500 mmHg x 21% = 105 mmHg.
Henry’s Law
When we inhale air all the major components of air are in gas form. However, the oxygen that enters our body must move from the air space in the lungs into the liquids found in our body such as blood plasma and cell cytoplasm. Alternatively, the carbon dioxide in the blood moves from the cell cytoplasm and the plasma into the air of the alveoli. To understand how the air-liquid interface affects the amount of oxygen or carbon dioxide found in the compartments of the body, we need to examine Henry’s law, which describes how gases dissolve in liquids based on their physical properties and the temperature of the liquid.
Henry’s Law explains that the amount of dissolved gas found in a liquid is proportionate to the gas phase partial pressure as well as the molecule’s solubility in a specific liquid. For example, considering partial pressures alone it would be expected that oxygen would readily diffuse from the aveoli because there is a high partial pressure gradient between alveoli and blood. However, oxygen has very low solubility in plasma, so even with the large gradient between the two very little of oxygen leaves the aveoli and dissolves directly into the plasma. Instead most of the dissolved oxygen found in the blood is attached to the hemoglobin molecules found inside the red blood cells. On the other hand carbon dioxide is very soluble in plasma (over 20 times more soluble than oxygen), so significant amounts can be found directly in dissolved form. Because of its high solubility, large amounts of carbon dioxide can move between air and liquid compartments even with small partial pressure gradients.
We can be significantly affected by differences in the partial pressures of the air we are breathing. At high altitudes, the fraction of oxygen in the atmosphere is the same (21%), but the total atmospheric pressure is less (as shown earlier). This causes the partial pressure of the oxygen we breathe in to be significantly less than that at sea level, making it much harder to move oxygen from the air into our plasma.
Decompression Sickness
Scuba divers involved in deep dives breathe in air that is under very high pressure. At sea level very little nitrogen enters our plasma since it has a very low partial pressure. However, a large amount of nitrogen can move into the plasma (and consequently the other body and cell compartments) during underwater dives since the air in the tank is under higher pressure as well as the diver who experiences the pressure of the column of water on their body. When the diver ascends slowly, the scuba apparatus automatically lowers the total pressure on the air the diver is breathing. The partial pressure of the nitrogen in the tank becomes less than the partial pressure of the nitrogen inside the diver’s body. Consequently the nitrogen that had moved from the air in the tank into the blood and tissues will now move from the blood and tissues back into the alveoli and be removed when the diver exhales.
But if the diver ascends too quickly, the gas, which has a very low solubility, comes out of solution and moves into the gas phase while still in the diver’s body. The gas phase becomes manifest as bubbles trapped in the diver’s tissues. The trapped bubbles can cause severe joint pains, dizziness, shortness of breath, extreme fatigue, paralysis, and possibly death. A hyperbaric (high pressure) chamber allows the diver to breathe air at an elevated partial pressure. The high partial pressure forces the nitrogen back into its dissolved liquid phase state. The barometric pressure of the chamber is then decreased very slowly to allow the nitrogen to be released slowly through the lungs.
Hyperbaric chambers can also be used to increase the partial pressure of oxygen and enhance its ability to enter our body through the respiratory system or through open wounds. Clinically high partial pressures of oxygen administered locally are used to treat people with regional infections such as bacterial gangrene infections sometimes found in the limbs of diabetics with poor blood circulation. Bacterial gangrene is an infection caused by anaerobic bacteria. These bacteria cannot survive in an oxygen-rich environment. The high partial pressures of oxygen found in the hyperbaric chamber can force more oxygen into the tissues infected by the bacteria. If the tissues in which the bacteria are living become too aerobic, the bacteria may die.

Exchange of Oxygen and Carbon Dioxide
External Respiration
Air containing oxygen enters the alveoli by the process of ventilation. The partial pressure of oxygen in the alveoli is slightly lower than the partial pressure of oxygen found in the atmosphere. Air taken into the lungs (tidal volume minus the volume of the conduction zone) mixes with air that is already in the lungs (functional residual volume). ). Because gas exchange is constantly occurring (even between breaths, when we hold our breath, etc.), the air making up the functional residual volume has had some oxygen removed from it and some carbon dioxide added to it. When a new tidal volume of air is inhaled, the portion of this air that enters the alveoli mixes with the functional residual volume and effectively lowers the fraction of oxygen in this alveolar air.
Inhaled air at sea level typically has a partial pressure of oxygen near 160 mmHg. In the alveoli, the partial pressure of oxygen would vary with our ventilation pattern, but typically equilibrates at about 105 mmHg. It is the difference between alveolar oxygen partial pressure and the plasma oxygen partial pressure that drives external respiration across the alveolar membrane. Blood coming through the pulmonary arterial circulation is lower in oxygen, with a typical partial pressure of 40 mmHg. The differential concentration gradient for oxygen to move from the alveolar air into the capillary blood starts at about 65 mmHg (105 mmHg in the alveoli minus 40 mmHg in blood), providing enough of a difference in partial pressures for oxygen to diffuse from the aveoli into the capillary. Diffusion will occur along the length of the pulmonary capillary until the partial pressures come into equilibrium near 105 mmHg.
The partial pressure of oxygen in the system circulation is slightly lower. As blood enters the left atrium of the heart, a small amount of the pulmonary blood mixes with the bronchial blood that has nourished the lung tissue, lowering the partial pressure so that the actual concentration of oxygen is about 100 mmHg, which is a typical value measured in a sample of arterial blood.
The functioning of the pressure gradient for carbon dioxide works in reverse of that for oxygen, with the carbon dioxide partial pressure being higher in the pulmonary arterial blood than in the alveoli. Even with a much smaller gradient for carbon dioxide than for oxygen, nearly as much carbon dioxide diffuses from the blood to the alveoli as oxygen diffuses from the alveoli to the blood because of the much higher solubility of carbon dioxide in the plasma.
Altitude Sickness
Altitude sickness, also called acute mountain sickness, can strike people climbing to elevations above 8,000 feet (although it typically occurs only at altitudes much higher than this). At elevations high above sea level, there is the same percentage of oxygen (21%), but much less atmospheric pressure. This lowers the partial pressure of the oxygen being inhaled so less oxygen enters the body. If the body doesn’t adapt well, a person can experience altitude sickness ranging from mild to severe forms. Mild to moderate altitude sickness can cause nausea, vomiting, tachycardia, shortness of breath with exercise, or difficulty sleeping. Mild to moderate cases usually resolve themselves when the person descends to a lower altitude. However, severe cases are another matter. They can result in cyanosis, pulmonary congestion, confusion and stupor, a cough with or without blood, a gray or very pale complexion, the inability to walk a straight line, if able to walk at all, and shortness of breath when at rest. These cases require immediate evacuation to lower altitudes. Without treatment, severe altitude sickness may result in death due to pulmonary complications or brain swelling. The good news is that altitude sickness can be prevented. Individuals who climb to extremely high altitudes, like Mount Everest, should do so slowly to allow their bodies to become acclimated to the atmospheric differences.
Internal Respiration
Internal respiration occurs between the blood and systemic tissues of the body. The systemic arteries carry essentially the same concentration of oxygen and carbon dioxide as the pulmonary veins. Oxygen is continually being used by the tissues, and the partial pressure of oxygen in active cells remains below 40 mmHg. Oxygen circulating in the systemic arterial blood readily diffuses across the membranes of the blood vessels into the tissues, replenishing the supply of oxygen in the cells. The final concentration of oxygen and carbon dioxide in the systemic veins is essentially the same as it is in the pulmonary arteries.
Just as the tissues are continually using up the oxygen, they are continually producing carbon dioxide. The partial pressure of carbon dioxide in tissues is always greater than 45 mmHg. This accounts for the diffusion of carbon dioxide into the systemic capillaries, raising the pressure to 45 mmHg. The systemic veins carry essentially the same concentration of oxygen and carbon dioxide as the pulmonary arteries.
| Internal respiration: tissues | PO2 | PCO2 |
|---|---|---|
| Systemic arteries leading to capillaries | 100 | 40 |
| Metabolically active tissues | < 40 | > 45 |
| Systemic veins | 40 | 45 |
Macromolecules of the Respiratory System
Mucus
Mucus is a slimy substance secreted by mucus membranes throughout the respiratory system and other organ systems. Mucus formation in the nasal passages and upper respiratory system, helps to moisten the air and to trap microorganisms and particles. Throughout the entire respiratory system, mucus helps humidify and buffer the cells that are in direct contact with air. Other organ systems including in the digestive, urogenital, visual, and auditory systems use mucus production to protect epithelial cells. While the molecular content varies between organ systems, in general, mucus contains primarily water with enzymes, immunoglobulins (and other immune proteins) salts, and high molecular weight glycoproteins called mucins. The glycosylations on the proteins attract large amounts of water. Consequently mucus serves as a means to maintain local levels of hydration.
Surfactant
At the gas-liquid interface of the alveoli cell membranes, surfactants found in the liquid surface layer lower surface tension. Surface tension arises when water molecules hydrogen bond with each other. The hydrogen bonding of water molecules makes it hard to “pull” the water molecules apart, which must happen for the alveoli to expand. If you have ever tried to pull two wet glass slides apart, you have experienced surface tension created by the hydrogen bonding of water molecules. The molecules in the surfactant interrupt these hydrogen bonds, making it much easier to expand the alveoli during inhalation. The composition of surfactant is 80% phospholipids with the remaining fraction made up of cholesterol and proteins. The alveoli themselves are so small that without the surfactant, the surface tension created by the water on the internal surface of the alveoli would force them to collapse during exhalation.
Respiratory Distress Syndrome of Newborn Infants
Respiratory distress syndrome of newborn infants (RDS) most commonly affects premature infants whose lungs have not developed fully, children born by caesarean section, and children of diabetic mothers. The lungs of these infants cannot make sufficient quantities of surfactant, making it very difficult for the affected infants’ lungs to expand properly to breathe. Most cases of RDS occur in infants born before 28 weeks gestation as the cells of the lungs responsible for surfactant production, called type II alveolar cells, are generally not very active until later in pregnancy.
Neonates with RDS struggle to breathe, leading to poorly oxygenated blood and cyanosis (appearance of blue skin). Additional symptoms generally include apnea (periods of breathing cessation) or rapid, shallow breathing. Laboratory procedures can be done to determine the level of fetal lung maturity.
Treatment for RDS often involves administration of a higher fraction of inhaled oxygen (above the normal 21%), or the use of a ventilator. Artificial surfactant can be given, but is still considered experimental. If a premature birth is likely, the expectant mother may be given a steroid such as cortisol to promote maturation of the fetal lungs before birth. Prognosis is dependent upon the severity of the disorder. Children often worsen within the first few days after birth, but then usually recover with appropriate treatment.
Hemoglobin
The protein used to carry oxygen by nearly all vertebrates is hemoglobin. It functions by binding oxygen in the capillaries of the lungs and carrying it inside red blood cells. When the blood enters capillaries in tissues with a low partial pressure of oxygen, the oxygen will leave the hemoglobin molecule and diffuse into the tissue. One gram of hemoglobin can carry 1.34 ml of oxygen under normal conditions. There are normally about 15 grams of hemoglobin in each deciliter of blood meaning that each deciliter of blood has the potential to carry about 20 ml of oxygen. This is much greater than the 0.3 ml of oxygen that a deciliter of blood can carry dissolved in plasma.
Hemoglobin is a complex compound containing two alpha and beta protein chain pairs and four heme molecules that contain iron in its ferrous (Fe+2) state. Each heme can bind one oxygen molecule, so a hemoglobin, because it contains 4 heme groups, can bind up to 4 oxygen molecules. When a hemoglobin molecule is fully bound (saturated) with oxygen, we consider it to be an oxyhemoglobin. When it is not fully saturated, it is typically referred to as deoxyhemoglobin, even though it may still have oxygen bound to some of the heme groups. Note that even though an individual hemoglobin molecule can only be 0%, 25%, 50%, 75% or 100% saturated, we have nearly 300 million hemoglobin molecules in each red blood cell. Collectively they can have any saturation level between 0% and 100%.
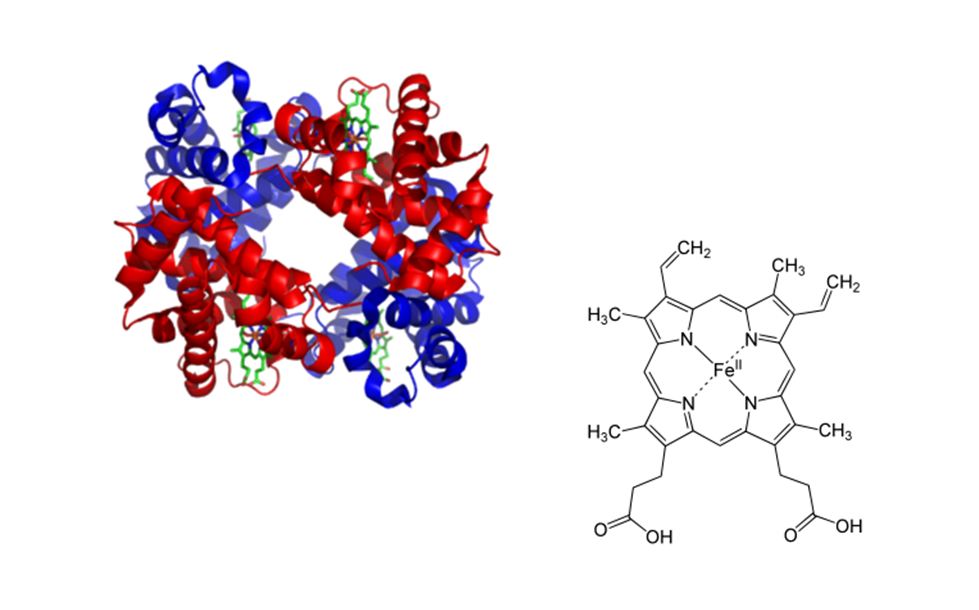
When oxygen binds to one heme group the affinity of the other binding sites changes so that the other three heme groups more easily bind each additional oxygen. Similarly, one heme group losing its oxygen makes it easier for the other three to lose theirs. This characteristic of heme facilitates loading and unloading of oxygen and helps to explain how oxygen can be so quickly attached and detached from hemoglobin as it passes through the appropriate capillaries.
Transport of Oxygen
Adult humans require about 250 ml of new oxygen to enter the bloodstream per minute to support the internal respiration of their tissues while in a relaxed state. Since oxygen is poorly soluble in our plasma, we only deliver about 15 ml of oxygen per minute to our tissues in dissolved form (0.3 ml of dissolved oxygen per deciliter of blood times 50 deciliters (a unit that refers to 10 mls of a liquid) per minute of cardiac output. Therefore we become critically dependent on another mechanism to help deliver sufficient amounts of oxygen to where it is needed. That mechanism utilizes a protein, hemoglobin as a transporter molecule for either oxygen or carbon dioxide. Red blood cells contain high concentrations of hemoglobin.
In order to be able to discuss the different macromolecules and the reactions they undergo, it is important to be familiar with the common chemical symbols that are used.
Here are a few of symbols you will see:
- Deoxyhemoglobin (HHb) – A hemoglobin molecule that has been reduced and does not have a full complement of oxygen molecules attached to it.
- Oxygen (O2) – A gas that is required for converting nutrients into cellular energy.
- Oxyhemoglobin (HbO2)- A hemoglobin molecule that has been oxidized and is bound to four oxygen molecules.
- Carbon dioxide (CO2) – A gas that is released as a waste product during the breakdown of glucose to release energy.
- 2,3-bisphosphoglycerate (BPG) – is a molecule found in red blood cells that can bind to hemoglobin and decrease its affinity for oxygen.
- Carbonic acid ( H2CO3 ) – is formed as an intermediate step in the transportation of carbon dioxide. Carbonic anhydrase is an enzyme that will speed up the formation of carbonic acid from water and carbon dioxide.
- Bicarbonate (HCO3−) – Carbonic acid can quickly convert to bicarbonate and a hydrogen ion. Bicarbonate plays a huge role in transporting carbon dioxide and maintaining blood pH.
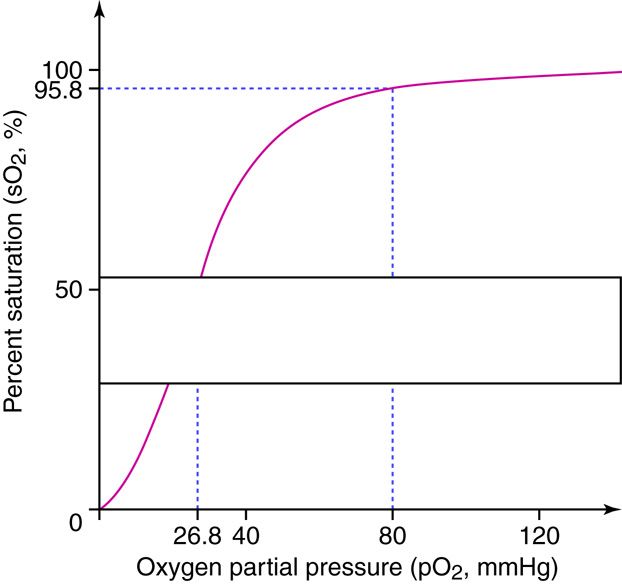
On this curve, the x axis lists the PO2 of the blood (whether it is in the lungs or other body tissues), and the y axis lists the percent saturation of the hemoglobin with oxygen. This graph has a sigmoidal ‘S’ shape, such that changes in partial pressures above 80 mmHg do not have a major effect on the percent of hemoglobin saturation. Since normal partial pressure in the lungs is over 100 mmHg, there are some safety factors that are important for people who travel to altitude or develop ventilation disorders to consider. As long as neither is too severe, hemoglobin will stay close to 100% saturated as long as the partial pressure in the alveoli can stay above 80 mmHg. Under normal conditions, hemoglobin ends up nearly 100% saturated in the lungs and then travels to the tissues. In the tissues, hemoglobin will have given up 25% of the oxygen it carries such that it is now 75% saturated (the relationship between oxygen levels and partial pressure is not linear because of the shape of the curve). If there is a higher demand for oxygen in metabolically active cells, more of the oxygen will diffuse into the tissues reducing hemoglobin saturation below 75%.
Hemoglobin and Oxygen Dissociation
Tight binding of oxygen to hemoglobin allows us to transport oxygen throughout our cardiovascular system. At the same time hemoglobin cannot bind oxygen so tightly that oxygen cannot leave the hemoglobin when it the molecule encounters oxygen deprived tissues. Metabolically active tissues cause local environmental changes making it easier for oxygen to unload from hemoglobin in these tissues. These changes in rates of dissociation can be visualized by looking at the oxygen-hemoglobin dissociation under different conditions such as changing temperature, PCO2, pH, and the levels of 2,3-bisphosphoglycerate (BPG). Increased cellular metabolism causes increases in temperature, BPG production and carbon dioxide levels. The increased carbon dioxide further causes a decrease pH. When this happens, oxygen more readily leaves hemoglobin molecules, and the oxygen-hemoglobin dissociation curve is shifted to the right. Regulation of oxygen delivery to cells is important since cells with a high metabolic rate need more oxygen to produce ATP.
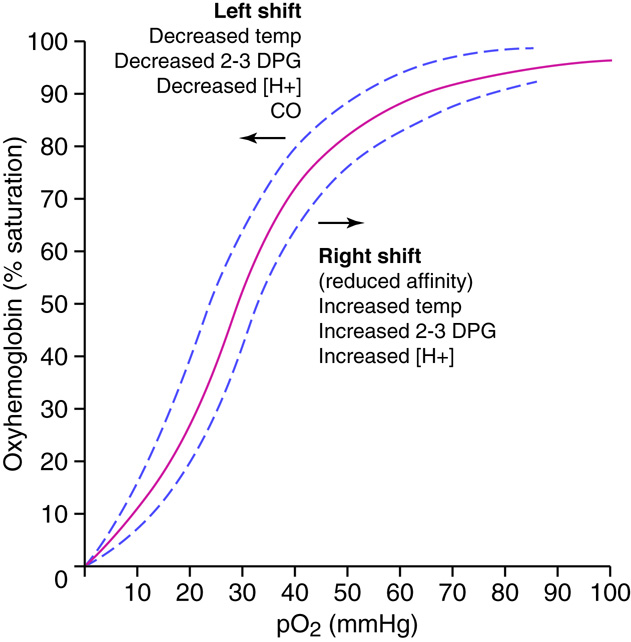
Specifically, the oxygen-hemoglobin bond is weakened in the more acidic environment, and the oxygen leaves the heme more readily. The most commonly found acids that result in a locally lowered pH include lactic acid and carbonic acid. Lactic acid production results from situations where there is inadequate oxygen supply and cells have shifted to anaerobic metabolism of glucose for energy production. Carbonic acid is formed when carbon dioxide dissolves in water. Since carbon dioxide production is a byproduct of aerobic metabolism, it follows that very metabolically active cells will exhibit increased levels of carbon dioxide production. The chemical equation linking the increased carbon dioxide to a lowering of pH is CO2 + H2O ↔ H2CO3 ↔ H++HCO3 which we will discuss later.
Red bloods metabolize glucose in a variation of the standard glycolysis pathway. BPG is an intermediate compound made in red blood cells during glycolysis. When present in red blood cells, it attaches to the terminal amino acid groups of hemoglobin’s beta chains and decreases the affinity of hemoglobin for oxygen. BPG will increase in response to endocrine regulators including thyroxine, epinephrine, norepinephrine, and testosterone, and as part of the compensation that occurs at high altitudes and with some anemias.
Transport of Carbon Dioxide
Carbon dioxide is carried in the blood in three forms: dissolved, attached to hemoglobin, and converted to bicarbonate ions. Dissolved CO2 accounts for 7–10 percent of the carbon dioxide carried in the blood. This is also the only form of carbon dioxide that diffuses from the tissues into the blood and from the blood into the alveoli for expulsion from the body. Here, we will examine in depth the transfer of carbon dioxide using hemoglobin or by the formation of bicarbonate.
Carbaminohemoglobin and Bicarbonate
Carbon dioxide can bind to any protein and form a carbamate compound. The protein found in the highest concentration in red blood cells is hemoglobin, and 20–23 percent of the CO2 carried in the blood is bound to hemoglobin in the form of carbaminohemoglobin. In the capillaries of the systemic tissues, CO2molecules attach to the terminal amino acids of the alpha and beta chains of the hemoglobin molecule. Deoxygenated hemoglobin (hemoglobin with no or less than the maximal oxygen bound, abbreviated HHb), such as that found in metabolically active tissues, binds CO2 easily. In the capillaries of the lungs, the elevated levels of oxygen found in alveoli force the carbon dioxide off the hemoglobin molecule and oxidize the protein, freeing up hydrogen ions. Although some carbon dioxide is transported as carbaminohemoglobin, the majority, about 70 percent, is dissolved in the blood as bicarbonate ions that arise from the reversible reactions discussed below.
Carbon dioxide in the presence of water can be reversibly converted to carbonic acid. Carbonic acid is not very stable and readily dissociates into a hydrogen ion and a bicarbonate ion. In fact, this is why carbonated beverages are acidic. Carbon dioxide is added to the drink mixture under pressure and dissolves in the beverage. When the CO2 has bubbled out of the beverage, it tastes flat because the acid is gone. The same thing happens in red blood cells, except that red blood cells contain an enzyme called carbonic anhydrase (CA), which is capable of facilitating one million reactions per second per enzyme molecule. Because of the enzyme, most of the CO2 dissolved in the blood is quickly converted to carbonic acid which breaks down to form, hydrogen ions, and bicarbonate ions.
The chemical reaction for this process is the following:
CO2– (in the presence of CA) + H2O ⇆ H2CO3 ⇆ H++ HCO3
Where H2O is water, CA is carbonic anhydrase, H2CO3 is carbonic acid, H+ is a hydrogen ion, and HCO3– is a bicarbonate ion. The second part of the reaction, which produces the hydrogen and bicarbonate ions, does not have an enzyme, but depends on the dissociation of the weak acid. This series of reactions provides buffering for the blood.
Carbon dioxide production occurs in many tissues, especially muscle. The carbon dioxide produced diffuses from the tissue of origin into a systemic capillary and dissolves in the plasma. A small amount of the carbon dioxide is transported this way. Most of the CO2 that diffused into the plasma diffuses into a red blood cell and reacts with intracellular water molecules to produce hydrogen and bicarbonate ions. Remembering that the reactions are reversible, it makes sense that the direction of the reaction sequences will be driven by levels of accumulated products. The dissociation of carbonic acid is driven by the relative concentration of carbonic acid compared to the relative levels of bicarbonate(carbonic acid’s conjugate base). A build-up of bicarbonate in the RBCs would slow or halt the dissociation of carbonic acid. This build-up doesn’t usually happen because, RBCs have a membrane channel that allows bicarbonate to leave the RBC and enter the blood plasma. To maintain electric neutrality inside the RBC and in the plasma, every time a negative bicarbonate ion leaves the red blood cell it is exchanged for a negative chloride ion from the plasma. This exchange is called the chloride shift. The bicarbonate ion in the plasma becomes part of the blood’s buffering system, maintaining blood pH within a narrow range. Deviation from this range compromises organ function and can cause death. The hydrogen ion liberated from the conversion of CO2 to bicarbonate binds to a a deoxygenated hemoglobin molecule causing it to become reduced. Deoxygenated hemoglobin easily picks up a molecule of CO2, creating carbaminohemoglobin. Hemoglobin is an important buffering agent for the hydrogen ions produced from the conversion of carbon dioxide to bicarbonate ions. If this buffering did not occur, the intracellular fluid of the red blood cell would become progressively more acidic, resulting in deterioration of cell functions. Some CO2 from the tissues can be found as as bicarbonate ions and dissolved CO2 in the plasma. The remainder of the carbon dioxide is attached to hemoglobin or it is still in the carbonic acid form and will stay in the red blood cells.
When the blood enters the pulmonary capillaries, gaseous carbon dioxide in the plasma diffuses into the alveoli. Some of the bicarbonate diffuses from the plasma into the red blood cells, and a chloride ion passes back into the plasma, reversing the chloride shift that occurred in the capillaries in the systemic tissues. The high partial pressure of oxygen in the alveoli causes the carbaminohemoglobin to dissociate into deoxyhemoglobin, a hydrogen ion, and a molecule of carbon dioxide. The released CO2 is available for diffusion. The free hydrogen ion combines with a bicarbonate ion and reforms carbonic acid. The carbonic acid is converted back to carbon dioxide and water under the influence of carbonic anhydrase.
Microscopic Level – Cells
Epithelial Cell Types
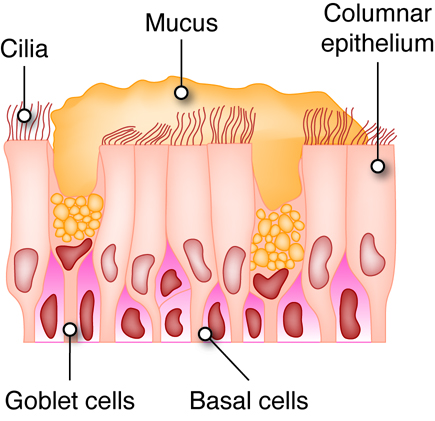
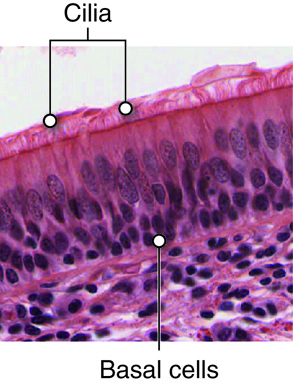
Respiratory epithelial cells include three types: a) ciliated cells, b) goblet cells, and c) basal cells. Ciliated cells have cilia on the surface, Goblet cells, shaped like a wine goblet, secrete mucus. Basal cells, found at the basal region of epithelial sheets, are stem cells used to regenerate and maintain a healthy epithelial cell layer.
Cilia
The apical surfaces of ciliated epithelia cells possess specialized structures called cilia. Depending upon the tissue cilia may function in either sensory or mechanical capacities. The cilia in the nasal cavity and bronchial tree sweep mucus toward the pharynx to be swallowed or expectorated. Swallowing delivers the microorganisms and particles that were trapped in the mucus to the stomach where the low pH of the stomach will destroy them.
Macrophages in the Lung
The respiratory system interacts with the external environment during gas exchange. This interaction can provide contact with pathogens (viruses, bacteria, and other disease causing organisms), atmospheric debris and other particulates. We have already covered how the sticky mucus traps many pathogens and particles and facilitates removal from the body. Yet the mucus does not trap all inhaled particles. Particles that make it to the level of the alveoli are typically removed by alveolar macrophages through the process of phagocytosis.
Alveolar Cells
In the alveoli there are specialized epithelial cells called alveolar cells. Type I alveolar cells are very thin, simple squamous cells through which gases easily diffuse. These cells are so thin that they can only be seen through the use of an electron microscope. The type I epithelial cells also make angiotensin-converting enzyme (ACE), an important enzyme of the renin-angiotensin system used in the control of blood pressure. Inhibition of this enzyme is one of the methods used to control hypertension. Scattered among the squamous cells are type II alveolar cells. Type II alveolar are cuboidal epithelial cells with microvilli on their apical surface. These cells make and secrete surfactant, which decreases the surface tension on the alveolar surfaces. The alveoli themselves are so thin that without the surfactant the force of surface tension created by the water on the cell surfaces would cause the alveoli to collapse upon exhalation. Mixed in among the types I and II alveolar cells are macrophages that migrate within the sacs and clean up material that has entered this part of the system. These cells may accumulate and sequester non-biodegradable material that persists in the cells. Cells that contain accumulated material are referred to as dust cells. The alveoli are considered to be functionally sterile, due to the combination of the mucus and cilia found through most of the respiratory membranes and the macrophages in the alveoli.
Epithelial Types Throughout the Respiratory System
In the nasal cavities and upper respiratory tract, epithelial cells are primarily ciliated psuedostratified columnar epithelium. The bronchial tree is lined with pseudostratified, ciliated, columnar epithelia with goblet cells dispersed among the columnar cells. At the terminal bronchioles, epithelial become ciliated cuboidal cells without any goblet cells.
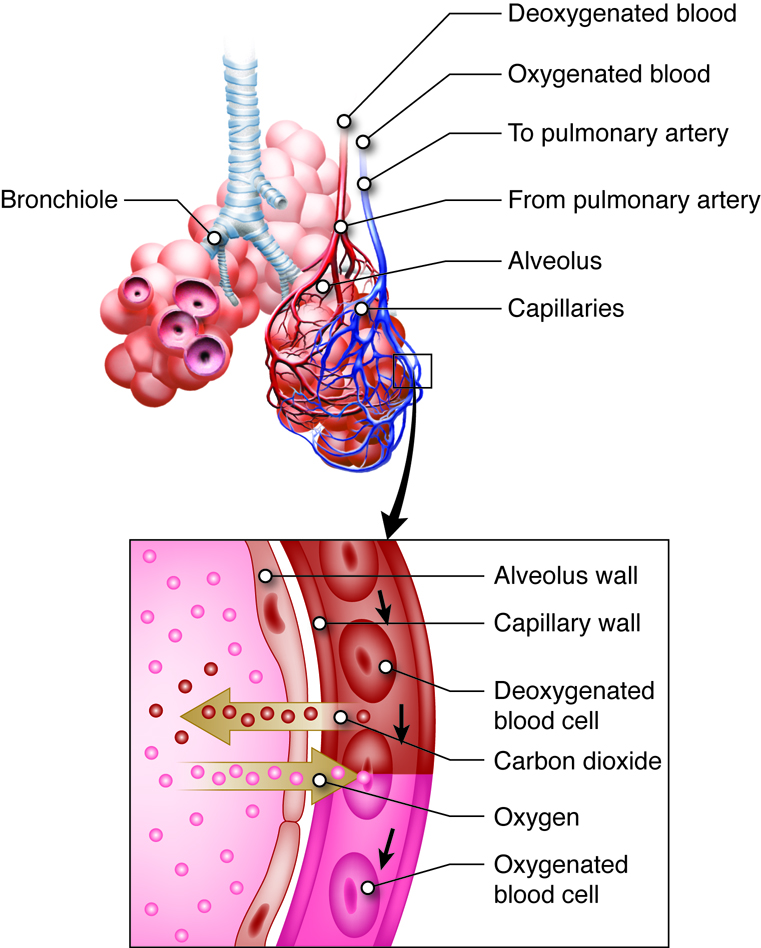
Anatomy of the Respiratory-Cardiovascular Junction
There is intimate contact between respiratory tissue and the blood supply in the lungs. Alveolar sacs in the lungs are wrapped in capillary beds of the cardiovascular system. At the cellular level, the simple squamous alveolar cells are in close contact with the capillaries, which are also one endothelial cell thick.
Oxygen from the inhaled air diffuses from the alveoli to the hemoglobin in the red blood cells. In order to do so, it has to diffuse through the alveolar epithelial cell, the capillary endothelial cell, plasma in the capillary, and into the red blood cell.
Respiratory Levels of Organization Summary
The major function of the respiratory system is to obtain oxygen needed to convert nutrients to energy and to remove carbon dioxide that is a waste product of this reaction. In order to perform this function the respiratory system must have mechanisms for moving oxygen into the body from the atmosphere and a way to transport the oxygen to tissues throughout the entire body. The respiratory system must also transport carbon dioxide to the lungs to be expelled from the body. Additionally, the respiratory system plays a role in protecting the body from microorganisms and maintaining proper pH levels.
The primary function of the respiratory system, the exchange of oxygen and carbon dioxide, occurs through several processes.
- Ventilation-moves air in and out of the lungs.
- External respiration exchanges oxygen and carbon dioxide between the air and the alveoli of the lungs.
- Internal respiration exchanges oxygen and carbon dioxide between the blood and tissues.
- Cellular respiration uses oxygen to release energy from nutrients.
- To understand how the respiratory system functions, it is studied on the chemical, macromolecular, cellular, tissue, organ and organ system levels.
- The role of partial pressure in the diffusion of gases in external and internal respiration
- Henry’s law discuss the solubility of gasses in a liquid and helps explain why oxygen and carbon dioxide are transported differently
- The macromolecule level related the structure of the macromolecules to its functions.
- Mucus- immunoglobulin for immune function, glycosylate proteins for hydration
- Surfactant- phospholipids that disrupt the surface tension of water in the alveoli to prevent collapse.
- Hemoglobin- iron containing heme group to transport oxygen and carbon dioxide.
- Transport of oxygen on hemoglobin- the benefit of a tight association and factors that will affect the affinity of hemoglobin for oxygen
- Transport of carbon dioxide as carbaminohemoglobin and bicarbonate as well as the role of carbonic anhydrase
- There are several important cell types that are found in different locations of the respiratory system.
- Epithelial cell types located in the respiratory tree include goblet cells that secrete mucus, ciliated cells that sweep mucus into the pharynx and basal cells which can regenerate and produce new layers of cells.
- Macrophages located in the lungs will help to provide protection against pathogens.
- Epithelial cells in the alveoli are divided into simple squamous Type I cells that allow for diffusion and also secrete angiotensin-converting enzyme and the cuboidal Type II cells that secrete surfactant.
In order to understand how the respiratory system functions, it is important to understand how the different organizational levels function both independently and together. Different portions of the respiratory system contain different cell types that all work together to allow for the diffusion of oxygen and carbon dioxide while providing protection from pathogens.

