43 Cardiovascular Levels of Organization
Capillary Exchange
The ultimate goal of the cardiovascular and respiratory systems is to deliver oxygen-rich and nutrient-rich blood to the capillary networks. Although the amount of blood within the capillaries at any given time is only about 5 percent of the total volume, it is the most important blood in the body. It is at the level of the capillaries that capillary exchange occurs. The thin and porous construction of capillary walls provides an avenue for substances such as oxygen and glucose to move out of the blood plasma and into the interstitial fluids surrounding the tissue cells. The reverse process, uptake of substances such as carbon dioxide and wastes from the interstitial fluids into the plasma, occurs simultaneously. In addition to the back-and-forth exchange of solutes, water also moves between the bloodstream and interstitial fluid in both directions within the capillary networks. There are three mechanisms by which substances enter and leave capillaries: diffusion, transcytosis, and bulk flow.
Diffusion
Diffusion, the movement of a solute from an area of high concentration to an area of low concentration, is a passive process. Diffusion is the driving force for the exchange of solutes at the capillary tissue interface. Thus, the oxygen and glucose, whose concentrations are high in the blood, will move out of the vessels into the interstitial fluids. Carbon dioxide levels are high in the interstitial fluid due to diffusion from the tissue cells. Carbon dioxide will move across the capillary walls into the blood plasma, where the concentration is lower. The physical characteristics of each solute determine the route by which that solute will diffuse. Small water-soluble molecules, such as glucose and amino acids, diffuse primarily through the intercellular clefts between the endothelial cells in continuous capillaries or through the fenestrations of fenestrated capillaries. Lipid-soluble molecules, such as oxygen and steroid hormones, can diffuse through the plasma membranes of the endothelial cells. Larger, lipid-insoluble substances must move down their concentration gradients via transcytosis.
Transcytosis
Transcytosis is a combination of endocytosis and exocytosis that is necessary when the substance to be transported cannot be exposed to the cytoplasm of an intervening cell. Transcytosis is important in moving large lipid-insoluble molecules such as proteins across the capillary endothelial cells. A protein, for instance albumin or insulin, which is high in concentration in the blood plasma, will be enclosed in tiny vesicles formed from the endothelial plasma membrane. This process is considered pinocytosis because the molecules dissolved in the blood plasma are taken up randomly along with the fluid. These molecules are transported within the vesicles across the endothelial cell. At the opposite plasma membrane surface, the vesicle merges with the membrane, and the molecules are released into the interstitial fluid. In the developing fetus, certain plasma proteins and antibodies are moved from maternal to fetal circulation via transcytosis.
Bulk Flow
Bulk flow is the movement of fluids due to pressure gradients. Fluids will move en masse from an area of high pressure to an area of low pressure. While diffusion is most important in the transfer of larger solutes, bulk flow controls the movement of fluids plus the ions and other small particles that are dissolved within the fluid. The movement of fluids from the capillaries into the interstitial fluid at the arterial end of the capillary bed is called filtration. The movement of fluids from the interstitial fluid into the capillaries at the venous end of the capillary bed is called reabsorption.
Filtration and Reabsorption
Bulk flow follows the same principles as any other type of pressure gradient. High pressure in one area promotes movement of fluid to an area of lower pressure. Capillary exchange is driven by two types of pressures. Physical or hydrostatic pressure is caused by fluid pressing against the confining vessel walls or other physical barrier. This is the same as the blood pressure measured within the capillary. Osmotic pressure is caused by the presence of non-diffusing solutes within the fluid. Nearly all small molecules, such as ions, freely diffuse across the capillary wall; as such, they don’t contribute to osmotic pressure differences between plasma and interstitial space like they do across cell membranes. Instead, it is the large plasma proteins that are responsible for the osmotic pressure differences across the capillary wall. The name given to the osmotic pressure caused by these proteins is colloid osmotic pressure. Depending on the location, the sum of the hydrostatic and colloid osmotic pressure, called the net filtration pressure (NFP), can promote filtration or reabsorption, or all the pressures can be at equilibrium and neither filtration nor reabsorption occurs. There are four pressures to consider when determining NFP, two on each side of the capillary wall: blood hydrostatic pressure (BHP), interstitial fluid hydrostatic pressure (IFHP), blood colloid osmotic pressure (BCOP), and interstitial fluid colloid osmotic pressure (IFCOP).
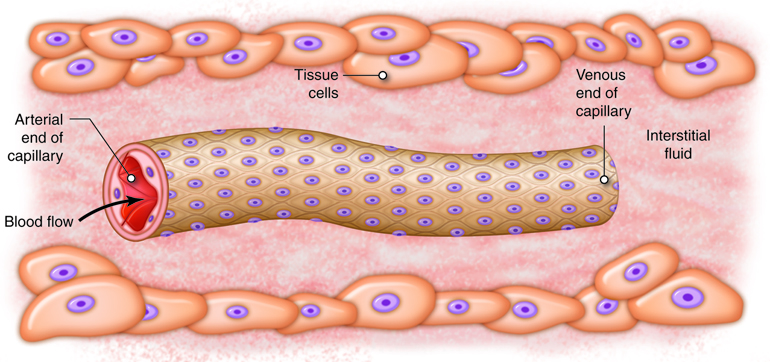
A high BHP promotes fluids moving from the capillaries into the interstitial fluid around the tissues. Alternatively, IFHP is very low throughout the tissues surrounding the capillary network. This is because local lymphatic vessels are constantly removing fluid from the interstitial space, keeping pressure very low. IFHP is so close to zero that it cannot counter the movement of fluid into the interstitial fluid under the effects of a high BHP. Due to this, IFHP is not typically considered in determining NFP. If there was not another force to counteract the hydrostatic pressure difference across the capillary wall, there would be a huge outflow of fluid from the capillary into the interstitial space. In order to provide balance and prevent this from happening, the high colloid osmotic pressure in the capillaries helps retain fluid in the vascular space. Most plasma proteins, such as albumin, fibrinogen and immune globulins are too large to diffuse from the bloodstream except at sinusoid capillaries. Therefore, the concentration of proteins or colloids is much greater in the capillaries than in the interstitial fluid. The result of this imbalance is an osmotic gradient that moves fluid from the interstitial fluid into the capillaries. The osmotic pressures show little variation along the length of the capillary network.
Disorders of Capillary Exchange: Edema
The accumulation of excess fluid in the tissues is called edema. There are various physiological causes of edema, including standing too long without moving your feet and legs, and pregnancy. Edema can also come about as a side effect of certain medications and can, in some cases, be a sign of a serious underlying medical condition, such as heart failure. Each cause leads to an imbalance in the rates of filtration and reabsorption during capillary exchange. When fluid is filtered from the capillaries more quickly than it can be reabsorbed, or if the lymphatic capillaries cannot remove normal amounts of interstitial fluid, the interstitial fluid begins to collect within the affected tissue or organ. If the volume of the interstitial fluid exceeds 30 percent above normal levels, edema can be detected as swelling of the affected area.
Most commonly, edema is caused by venous congestion (increased venous pressures) caused by increased resistance to blood flow through major veins, reduced pumping ability of the right heart, or excessive fluid levels in the body. Pregnancy and liver disease can make it harder for blood to return through the venous system from the legs or abdomen, respectively. In each case edema will occur in the tissues with impeded flow. Pregnancy commonly causes edema in the legs, while liver disease causes abdominal edema, called ascites. Right heart failure leads to a more generalized edema, as venous flow from the entire body is affected. In each case, BHP is greatly increased within the capillaries. Because BHP is the driving force of filtration, the rate of fluid leaving the capillaries escalates with increasing BHP. High BHP also opposes reabsorption. Despite the rise in IFHP due to extra fluid within the tissues, the high BHP results in less fluid reabsorption at the venous end of the capillary network. A high BHP also puts uncommon pressure on the walls of the capillaries, forcing the epithelial cells apart, and the intercellular gaps become wider. Plasma proteins normally confined to the blood vessels spill into the interstitial fluid. This causes IFOP to increase and BCOP to decrease, causing fluid to accumulate even more rapidly. Edema can only be resolved by intervention to prevent the filtration/reabsorption imbalance and time for the lymph system to collect the excess fluids.
Hemostasis and Blood Clotting
Hemostasis (hemo– blood and stasis – standing still) is a protective process in which the body undergoes a cascade of steps in order to stop the bleeding from a damaged blood vessel. The process of hemostasis is incredibly complex so that blood clots form where and when we need them to. We have all seen that small cuts on our skin begin the healing process with a clot. However, clots are not always beneficial. The formation of blood clots within an intact blood vessel can lead to heart attacks and strokes. Thus, this process has to be well regulated. Hemostasis is a multi-step process, characterized by three major activities: vasoconstriction, platelet aggregation, and formation of a fibrin clot.
When a blood vessel is torn, the first response is a vascular (vascular refers to blood vessels) spasm, which is the constriction of the blood vessel at its site of injury. This vascular constriction is initiated by activation of pain receptors that reflexively cause constriction of local smooth muscle cells.
Second, platelets (a type of cell fragment) stick to the collagen fibers that become exposed when the endothelial cell lining of the blood vessels is damaged during injury. In doing so, they begin to plug up the hole. These bound platelets also become activated and release secretory granules that contain adenosine diphosphate (ADP), serotonin, prostaglandins, and phospholipids. These signaling molecules lead to binding of more platelets at the site of injury. This process continues until enough platelets have aggregated to form a platelet plug. Serotonin, a neurotransmitter, and thromboxane A2, a prostaglandin produced by activated platelets, both facilitate vasoconstriction at the site. The released phospholipids activate the clotting factors. In the end, the platelets at the site of injury form of loose meshwork of interconnected platelets.
Third, a more stable plug called a clot, or thrombus, forms via coagulation (blood clotting). Coagulation occurs as a series of reactions that result in the formation of the fibrin protein. This fibrin protein can cross-link to other fibrin proteins, as well as to platelets, providing strength to the thrombus. The end product of coagulation is a clot, or thrombus, consisting of a fibrinous gel that encloses trapped blood cells. During the coagulation process the clotting cascade amplifies itself, making this a classic example of a homeostatic feedback loop that uses positive feedback (although in a limited manner).
Fibrin is formed from the plasma protein fibrinogen. This conversion occurs only after a cascade of enzymatic reactions involving various plasma proteins called clotting factors. The 13 clotting factors are named in the order of their discovery as I through XIII. Two separate pathways exist in the clotting pathway: an extrinsic and intrinsic pathway. The majority of the reactions that occur in both the extrinsic and intrinsic pathways require calcium in order for the steps to occur. Coagulation can be inhibited by adding a chelating agent, such as citrate, that binds to calcium making it unavailable to facilitate blood clotting. This is a way to prevent freshly drawn blood from clotting.
Problems in Blood Clotting
Normal hemostasis can be reduced by a number of conditions, including decreased platelet levels, genetic defects leading to insufficient or abnormal clotting factors (hemophilias), or lack of production of clotting factors in the liver (where many are formed). Certain clotting factors require vitamin K, a fat-soluble nutrient that is synthesized by bacteria in the large intestine. Conditions that result in the malabsorption of fat lead to the deficiency of vitamin K and correspondingly increased bleeding times.
Blood clots can break free from sites of injury. These thromboemboli then get trapped within small blood vessels, blocking flow through the vessels. Thromboemboli that originate in the venous circulation travel until they reach the small blood vessels in the lungs where they lodge as pulmonary emboli. If these thromboemboli are large enough, the person will have abnormal breathing or may die.
Blood Cells
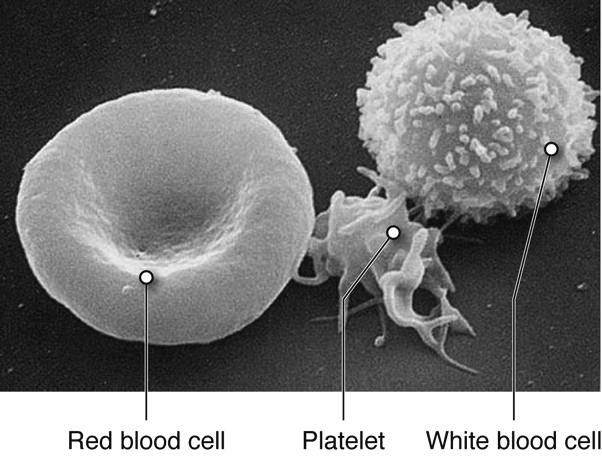
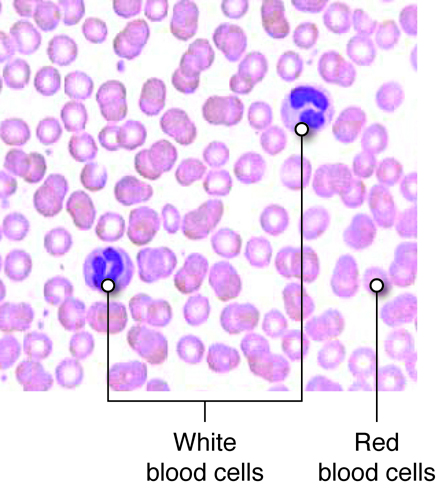
There are three main types of blood cells that flow through the blood stream, with each type serving specialized functions. Red blood cells primarily carry oxygen and remove carbon dioxide, white blood cells are immune cells that patrol the blood and body tissues for pathogens and platelets, which are involved in hemostasis, as described previously.
Red blood cells (RBCs) are one of the most abundant formed elements in blood. Also called erythrocytes, red blood cells are specialized for oxygen transport and function primarily in gas exchange. Red blood cells bring oxygen to tissues and pick up carbon dioxide (the gaseous waste product of cells), bringing it back to the lungs. Red blood cells contain hemoglobin, a protein that contains iron, to which both oxygen and carbon dioxide are able to bind. In the lungs, red blood cells pick up the oxygen that they transport.
The various types of white blood cells (WBCs), or leukocytes, are readily distinguished by whether or not they possess granules within their cytoplasm. Granulocytes, as their name suggests, possess numerous granules in their cytoplasm, whereas agranulocytes do not. The shapes of the nuclei of granulocytes and agranulocytes also differ. Leukocytes mainly function to protect the body from microbial invasion and toxins. WBCs readily travel through the bloodstream. However, most leukocytes can also travel from the bloodstream into the interstitial tissues in order to attack foreign agents in those spaces.
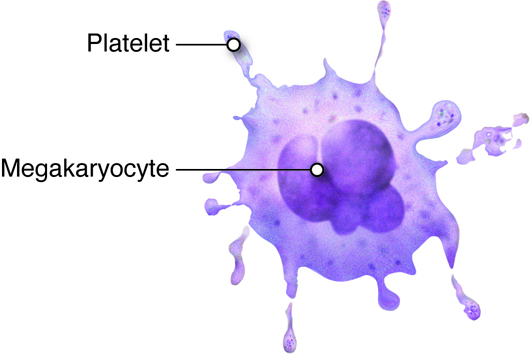
Platelets are smaller fragments of larger cells called megakaryocytes, and they are sometimes not even classified as cells. Megakaryocytes are large nucleated cells off of which numerous platelets bud. Platelets lack a nucleus but do have a plasma membrane surrounding a cytoplasm and a few organelles. As described previously, platelets play a role in hemostasis.
Red Blood Cells
Red blood cells (RBCs), also called erythrocytes (erythro– red and cytes –cells), are disc like cells that are the most abundant cell types in blood. Red blood cells transport the oxygen from our lungs to all of our body’s cells that carry out aerobic respiration. RBCs also pick up carbon dioxide (the gaseous waste product of cells) from metabolically active cells, bringing it back to the lungs. Red blood cells contain a special protein that contains iron, called hemoglobin (Hbg), to which both oxygen and carbon dioxide are able to bind. Red blood cells do not have nuclei or many other organelles, which leaves more room in cytoplasm for hemoglobin needed for the cell’s job to transport these molecules.
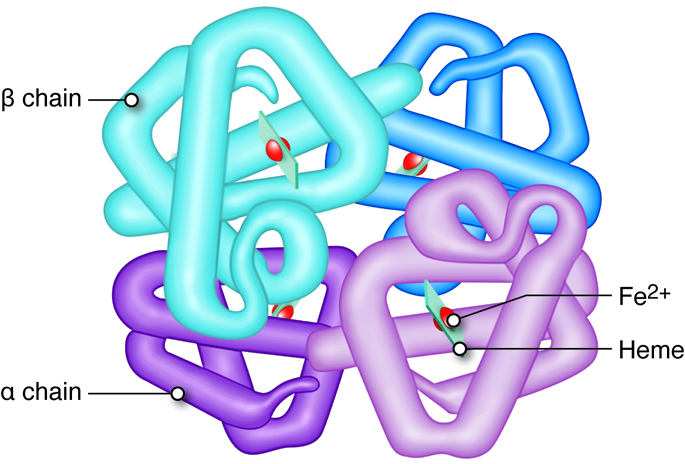
Hemoglobin consists of four heme groups (non-protein) and four globin (protein) molecules. Each heme group is composed of an iron structure surrounded by carbon rings containing nitrogen. Heme, in combination with oxygen, is responsible for the red pigment of blood. Each hemoglobin molecule can transport up to four oxygen atoms, one bound to each heme group, when the hemoglobin is said to be fully saturated. In fetuses and infants, hemoglobin is mainly composed of two alpha and two gamma globin chains (there are very few beta chains). As an infant develops, the gamma chains are replaced by beta globin chains. In an adult, hemoglobin consists of two alpha and two beta globin chains. A single erythrocyte has about a quarter of a billion hemoglobin molecules, which demonstrates the amazing capacity of red blood cells to transport oxygen.
Blood passing through the lungs is exposed to high oxygen levels, and when red blood cells pick up this oxygen the hemoglobin is called oxyhemoglobin (HbO2). As blood passes through other tissues, oxygen-carrying RBCs drop off a fraction of this oxygen (typically 25%). The hemoglobins that are no longer carrying oxygen are called deoxyhemoglobin (Hb).
If hemoglobin has lost a fraction of its bound oxygen, then a small amount of carbon dioxide can bind to the protein sections of Hb (versus oxygen that binds to the heme), and can be transported from tissues to the lungs. This hemoglobin is referred to as carbaminohemoglobin (HbCO2), which accounts for 25 percent of the carbon dioxide transported in in the blood. However, the bulk of carbon dioxide collected from tissues throughout the body is transported in the form of bicarbonate (HCO3–) in the plasma. The enzyme carbonic anhydrase, found in high concentration in erythrocytes, catalyzes the conversion of carbon dioxide and water into bicarbonate. This bicarbonate then moves into the plasma in an exchange with chloride across the RBC membrane.
Life Cycle of Red Blood Cells
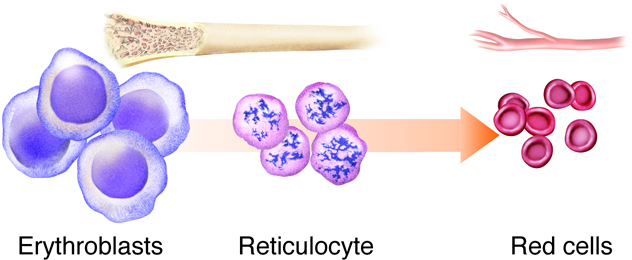
Erythropoiesis is the process by which bone marrow stem cells develop into erythroblasts that differentiate further into reticulocytes, and then mature before being released into the circulation (Figure 8). This process of making RBCs occurs continuously and at a high rate, with approximately 2.5 million RBCs produced every second. This matches the breakdown rate of RBCs, as old ones are removed and destroyed by the liver and spleen. Erythropoietin is the hormone from the liver and kidney that regulates the rate of RBC production.
Mature red blood cells are disc-shaped structures with a flattened center. They do not possess a nucleus or other organelles, but instead use the space for hemoglobin. The depressed center also maximizes the surface area of red blood cells, which facilitates the exchange of O2 and CO2. The flattened shape of RBCs allows them to navigate through narrow capillaries, which are often of similar (or smaller) diameter than the RBCs. The lack of a nucleus also means that RBCs lack the genetic material (DNA) necessary to reproduce, grow, and carry out functions of maintenance and repair. This limits the lifespan of these cells.
Red blood cells have a typical lifespan of 120 days. Over time, red blood cells accumulate cellular damage. Once they get to a point where they cannot deform appropriately when moving through narrow channels, they are removed from the circulation by specialized cells, called macrophages, in the spleen or liver. Macrophages phagocytose (engulf) the old RBCs, separating the protein (globin) and non-protein (heme) portions of hemoglobin. The protein chains are further degraded into amino acids and dispersed within the blood plasma. Iron is extracted from heme and is either stored or enters the bloodstream. Stored iron is attached to proteins ferritin and hemosiderin, preventing the buildup of toxic free iron. Iron entering the bloodstream is also attached to a molecule called a transferrin; which can be picked up for use by various tissue types such as muscle cells, or even bone marrow, where it is used to generate new erythrocytes.
Blood Groups
You are probably aware that if an individual is transfused with non-compatible blood that negative consequence, such as the clumping together (agglutination) or destruction (hemolysis), of the donated red cells might result. Compatibility is determined by the lack of interaction between antigens on the donor red blood cells and antibodies in the plasma of the recipient. People of different blood groups (types) vary in the red blood cell antigens and plasma antibodies they have.
Although there are many blood groups, the ones that are the most important when it comes to transfusions are the ABO and Rh groups. These groups are genetically determined, with a single gene coding for protein synthesis being responsible for each. The alleles inherited determine which if any protein antigen(s) (sometimes called agglutinogens) are expressed and embedded in the RBC membrane. In the case of the ABO blood group, the two alleles of the gene can each be one of 3 different variants, O (i), A (IA), or B (IB). The A and B alleles are dominant to the O allele, while the A and B alleles (AB or IAIB genotype) are codominant, creating the AB blood group when inherited together. The O or null allele results in no antigen being created (so A blood group could be either AA or AO genotype). For the Rh group the dominant D allele determines if a person is Rh+ with possible genotypes of either DD, Dd, or dd.
Note that these two blood groups are distinct from one another such that they are used in combination, and not one or the other. For example, those with blood type O- express neither antigen A or B or D, while those with type A+ blood express the A antigen and the D antigen on their red cells.
Besides the antigens, the ABO blood group also determines the antibodies (sometimes called agglutinins) that are present in the person’s plasma. These antibodies start forming soon after birth, likely in response to bacteria with similar membrane antigens that start inhabiting the gut. Those with type O blood will make both anti-A and anti-B antibodies, those with type AB blood will not produce either antibody, while those with either type A or type B blood will make antibodies against the antigen not found on their red cells. In contrast, those that are Rh-, do not make anti-D antibodies unless exposed to a significant amount of blood containing the D antigen.
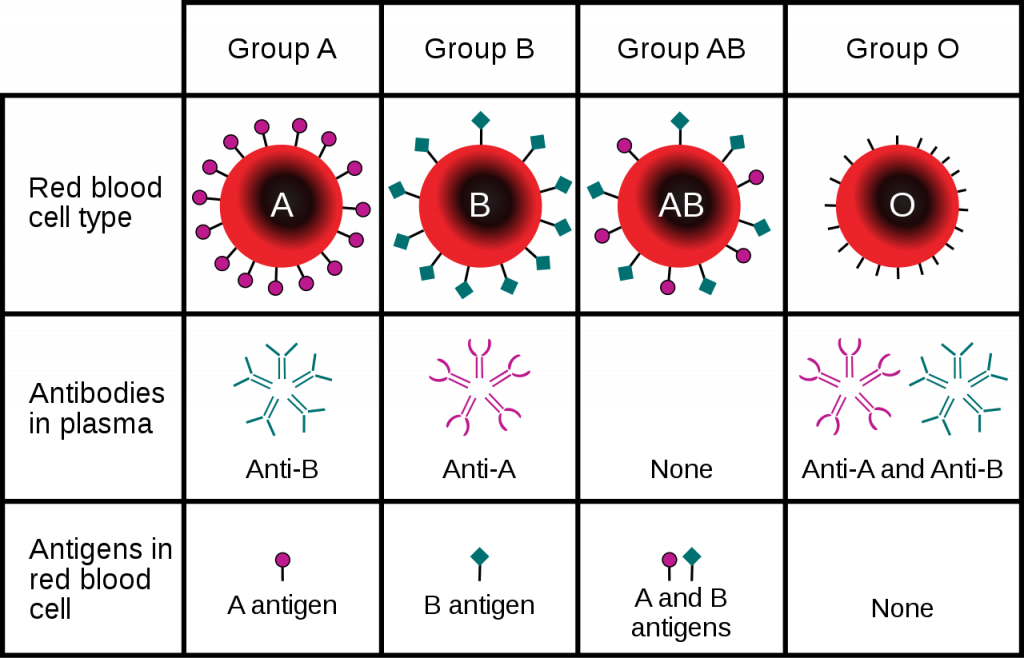
As mentioned, in order for a blood transfusion to be compatible, the donor blood cannot contain antigens that will cross react with recipient plasma antibodies. The most obvious way to prevent transfusion reactions is to infuse donor red blood cells (which is more common than using whole blood) that are of the same type as the recipients. But in some cases other red blood cells can be used as well. For example, because those individuals who are blood type AB do not have any anti-A or anti-B antibodies in their plasma, they can accept blood from any other ABO blood type (as long as the RH factor matches as well), making them the universal acceptor.
Red Blood Cell Disorders
Anemia
Anemia is a condition in which the oxygen carrying capacity of the blood is reduced. It is indicated by a low hemoglobin concentration, and typically presents with a decrease in the number of red blood cells.
| Types of Anemia | Clinical Features | Origin of Anemia |
|---|---|---|
| Iron deficiency anemia | Failure to synthesize adequate amounts of RBCs | Poor dietary intake of iron, blood loss |
| Pernicious anemia | Failure to synthesize adequate amounts of RBCs | Poor intestinal absorption of B12, a vitamin necessary for RBC production |
| Folic acid deficiency anemia | Failure to synthesize adequate amounts of RBCs | Poor intake of dietary folic acid; inability to absorb folic acid; medications (metformin is a diabetic drug that can interferes with folic acid utilization) |
| Hemolytic anemia | Rapid degradation of RBCs | Genetic or acquired diseases that alter the shape of RBCs; toxins or drugs |
| Sickle cell anemia | A form of hemolytic anemia in which RBCs are degraded after they assume a sickled shape. When sickled, RBCs clump together and block blood vessels. | Inherited disease in which RBCs develop a sickle shape;most common among African Americans |
| Thalassemias | A form of hemolytic anemia resulting from a family of disorders associated with abnormal hemoglobin. There are several variants of the disease depending on if it is the alpha or beta chains that are affected, and whether the individual is homozygous or heterozygous for an abnormal gene. | Inherited diseases that are most common in individuals from Greek, Italian, Asian, African or Middle Eastern descent |
White Blood Cells
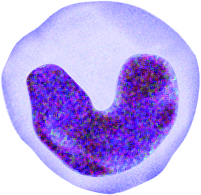
White blood cells, or leukocytes, are immune cells that are present in the blood. The detailed mechanisms of immune function are covered in the immunity unit, but we will discuss the classes here. There are five common types of leukocytes (and some of those types have subgroups). One way of categorizing these five is by whether or not they contain granules in their cytoplasm when the cells are stained. If they do, they are a type of granulocyte. If not, they fall into the category of agranulocytes. Another way of distinguishing leukocytes from one another is by the morphology (shape and patten) of their nuclei. Granulocytes have multilobulated nuclei and agranulocytes have a spherical (nonlobulated) nucleus. The lifespan of a granulocyte ranges from about 12 hours to 4 days, but the agranulocyte survives for approximately 100 to 300 days.
Leukocytes mainly function to protect the body from microbial invasion and toxins. WBCs readily travel through the bloodstream. However, certain leukocytes have the ability to move into the interstitial spaces of the body’s tissues in order to attack foreign agents to protect the body from infection. They leave the capillalry by squeezing through the pores in the capillary walls via a process called diapedesis. This is due to the ability of these leukocytes to rearrange cytoskeletal matrix in a manner that changes their shape.
Granulocytes
Granulocytes contain many granules within the cytoplasm, and they have a multilobular, irregularly shaped nucleus. There are three types of granulocytes: neutrophils, eosinophils, and basophils. Each granulocyte is identified by specific stains used to reveal the granules in their cytoplasm. Granulocytes are responders of the nonspecific immune system, which means that the cells respond to injury and build up an inflammatory response against microbes or toxins regardless of whether the body has been previously invaded by the foreign agent. That is to say, granulocytes do not possess memory when mounting an immune response.
| Neutrophil | 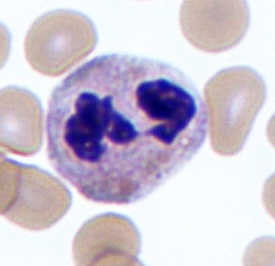 |
 |
| Eosinophil | 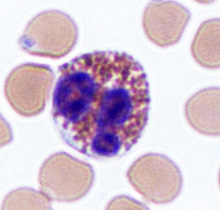 |
 |
| Basophil | 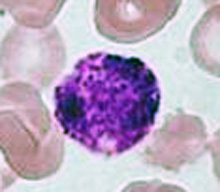 |
 |
| Monocyte | 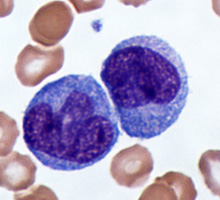 |
 |
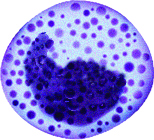
Neutrophils
Neutrophils are the most abundant type of granulocyte (50 to 70 percent of circulating WBCs). The multi-lobular shape of the nucleus often classifies neutrophils as polymorphonuclear leukocytes (PMNs). During a bacterial infection, neutrophils are the first responders. The PMNs arrive at the site of infection through a process known as chemotaxis in which chemicals released by damaged cells draw neutrophils. The neutrophils attack bacteria by engulfing them via the process of phagocytosis. Once the bacteria are contained within the neutrophils, the granules release antimicrobial compounds called defensins and lysozymes to destroy the microbes. Pus is the culmination of destroyed neutrophils, bacteria, and dead tissue.
Eosinophils
Unlike neutrophils, eosinophils stain red with an eosin dye; the dye is acidic and is picked up by the cytoplasmic granules that contain digestive enzymes. Eosinophils also engulf foreign molecules attacking the body, particularly in parasitic infection and allergic reactions. They also play a role in modulating the activity of basophils.
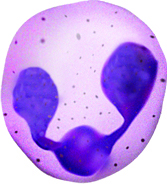
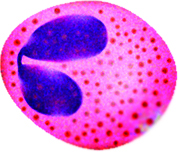
Basophils
The nucleus of basophils can contain two to five U- or S-shaped lobules. These leukocytes pick up basic dyes that cause their granules to have a blue-purple appearance. The granules of basophils contain histamine, serotonin, and heparin. The cells release histamine during an inflammatory response to tissue damage and microbial invasion. Basophils are similar in appearance and function with mast cells, which are strictly localized in connective tissue.
Agranulocytes
Unlike granulocytes, agranulocytes do not have granules within their cytoplasm and lack a lobulated nucleus. Under a microscope agranulocytes are observed to have a nucleus that composes the bulk of its cellular volume. Agranulocytes include lymphocytes, which are responsible for the specific immune response, meaning that they have memory and build up a vigorous response against toxins or microorganisms that the body has encountered before. The other agranulocyte is the monocyte, which is an immature form of the non-specific macrophage.
Lymphocytes
Lymphocytes can be categorized by their size (small, medium, and large). These cells have a round nucleus that virtually fills up the cell leaving very little blue-staining cytoplasm surrounding it (when stained in a standard manner). Lymphocytes are the only WBCs that reenter the bloodstream after moving into tissue to attack foreign agents.
There are two types of lymphocytes: T lymphocytes (T cells) and B lymphocytes (B cells).
They both originate in the bone marrow, but T cells grow and mature within the thymus gland while B cells typically mature in bone marrow then move to lymph nodes and similar tissue. T cells attack abnormal cells in the body, such as virally infected cells, tumor cells, and donor transplant cells. B cells sense and attack invading antigens (foreign substance in the body) such as toxins, viruses, or bacteria. In response to antigens, B cells divide into plasma cells that make antibodies aimed at neutralizing and destroying these antigens.
Monocytes
The nuclei of monocytes are also large, but have a kidney shape. The cytoplasm of monocytes is greater in quantity compared to lymphocytes, and it stains in a bluish-gray color. Monocytes have the capacity to leave the bloodstream and enter tissue, where they mature into cells called macrophages. Macrophages phagocytose (phagocytize) microbes and tissue debris.
White Blood Cell Disorders
There are three types of disorders that affect WBCs: leukocytosis, leukopenia, and leukemia. Leukocytosis is a condition characterized by an abnormally high number of mature WBCs. A common type of leukocytic illness is mononucleosis, in which monocyte numbers greatly increase as a result of a viral infection. Signs of this condition include enlarged spleen and lymph nodes. Leukopenia is an abnormally low level of WBCs and commonly occurs with the use of steroids such as cortisone. Like leukocytosis, leukemia is also characterized by a high number of WBCs; however, this high number is a result of cancerous proliferation of immature, nonfunctional WBCs.
Neutropenia is a condition in which neutrophils are abnormally low in the body. Because these granulocytes play an important role in fighting infection, individuals with neutropenia are more susceptible to infections. These infections are also more likely to develop into life-threatening conditions. Neutropenia often occurs in people undergoing chemotherapy or radiation therapy. Physicians may suspect neutropenia in patients who frequently develop infections.
Platelets
Platelets, as we described earlier are primarily involved in hemostasis. Platelets are smaller fragments of larger cells called megakaryocytes. Megakaryocytes are large nucleated cells off of which numerous platelets bud. Platelets, like red blood cells, also lack a nucleus. They do, however, have a plasma membrane surrounding a cytoplasm and a few organelles. Platelets are the smallest formed elements of blood with a diameter of only 1 to 2 micrometers. Platelets are the cellular component in the process of hemostasis in which a clot is ultimately formed to stop blood loss from a damaged blood vessel. Under normal circumstances, platelets are repeled by the endothelial lining of blood vessels. This is due to the endothelial cells releasing the prostaglandin prostacyclin. Upon vascular injury prostacyclin levels fall, and, along with exposed collagen as a binding site, platelets start adhering to the damaged area. Through a series of further steps, platelets and other proteins will seal the damaged blood vessel wall by forming a blood clot.
Hematopoiesis and Stem Cells
Hematopoiesis, or the formation of blood cells, occurs in the red bone marrow of the body. Recall that seven different cell types (including platelets) circulate in the blood, yet their lineage can all be traced to stem cells in the bone marrow. As these stem cells produce new cells via mitosis, the daughter cells may follow different maturational paths, depending on the needs of the body. Two common paths are the myeloid cell line and the lymphoid cell line. The myeloid line gives rise to red blood cells, granular leukocytes, platelets, and monocytes. The lymphoid cell line produces T and B lymphocytes. Although most myeloid cells mature in the myeloid tissues of the red bone marrow of the skull, vertebrae and rib cage (as well as long bones in children), lymphoid derived cells typically mature in lymphoid tissues throughout the body, such as the spleen, tonsils and lymph nodes.
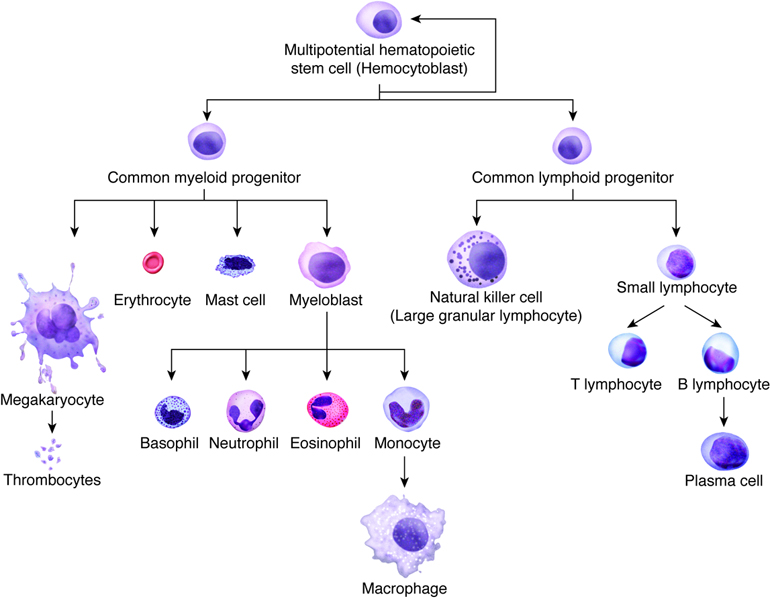
During embryonic development the formation of blood cells occurs in the liver, spleen, and yolk sac. However, after birth, the infant’s liver and spleen become the locations for destroying blood cells. All blood cells, whether they end up in the myeloid or lymphoid family, begin as hemocytoblasts, stem cells that develop from embryonic mesenchyme.
Formation of the Seven Cell Types in the Blood
| Progenitor Cell | Formed Element | Function |
|---|---|---|
| Proerythroblasts | Red blood cells | Transport oxygen and carbon dioxide |
| Myeloblasts | Neutrophils | Phagocytic |
| Basophils | Release heparin (anticoagulant) | |
| Eosinophils | Phagocytic | |
| Lymphoblasts | Lymphocytes | Provide specific immune response |
| Monoblasts | Monocytes | Phagocytic |
| Megakaryoblasts | Platelets (thrombocytes) | Hemostasis |
Cells of the Vessels
Within a blood vessel there are three main layers, which we will consider in vascular tissue, described later. Endothelial cells are on the inside (lumen) of a blood vessel. In some vessels, they are wrapped by smooth muscle cells. Fibroblasts secrete extracellular matrix in the large vessels, creating a connective tissue outer layer.
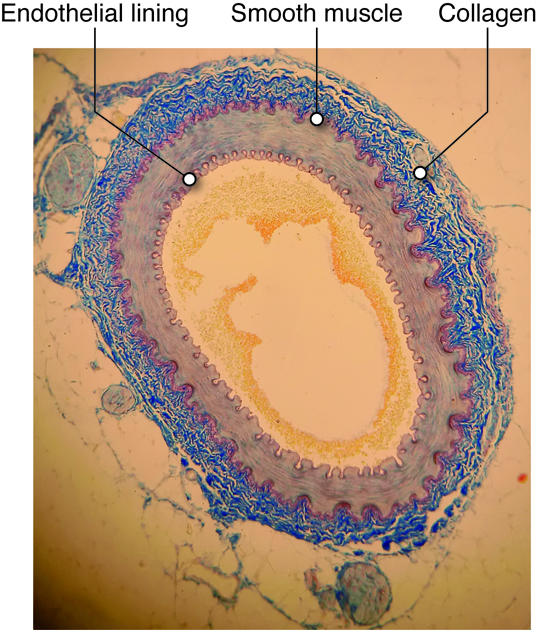
Endothelial Cells
Any surface that comes in contact with blood, including the inside of the heart and every blood vessel, is lined with a special cell called an endothelial (endo-inside) cell. Vascular endothelium line the blood vessels, and there are special endothelial cells of the lymph system. Endothelium of the interior surfaces of the heart chambers are called endocardium.
The layer of endothelium is only one layer thick, and these cells have several important functions.
Mechanical-chemical Stimulation
Endothelial cells sense changes in local chemical and mechanical environment, and signal to the underlying muscle cells. For example, under certain conditions, increased force on the vessels cause endothelial cells to release vasodilator chemicals (such as nitric oxide) which cause the smooth muscle cells to relax, leading to a dilated (expanded) vessel.
Immune Response
When tissues have been compromised by damage or invasion of pathogens, the immune system releases chemical signals (cytokines) into the region. These cytokines activate the endothelial cells to promote association with circulating immune cells. Also, pores open up between endothelial cells to allow immune cells from the circulation to leave the lumen of the vessel and invade the tissue.
Smooth Muscle Cells
Smooth muscle tissue is found throughout the body, including around organs in the digestive, respiratory, and reproductive tracts and around blood vessels. In the cardiovascular system, smooth muscle cells form a layer outside of the endothelial cells. Here they provide strength to blood vessels and provide a mechanism for vessel adaptation.
Smooth muscle cells contract via the actin-myosin contraction mechanism described in the muscular unit. Smooth muscle cells adjust their contraction based on sensed stretch or the concentration of various chemical factors. For example, hormones related to exercise or anxiety can cause vessel constriction and nitric oxide can cause vasodilation.
Extra-Cellular Matrix of the Vessels
Besides the endothelial lining found in all blood vessels, and the smooth muscle cell layer found in most vessels, all vessels except capillaries also have one or more layers of connective tissue associated with them. These vessels have an outer connective tissue layer (tunica externa, or adventitia), while some also have a thin layer of connective tissue, called the internal elastic lamina that is found between the endothelial layer and smooth muscle layer. The types and concentrations of molecules in these connective tissue layers provide structure, support, and determine how easily the vessels will passively (without relaxation of muscle cells) expand.
Elastin
Even without active changes in smooth muscle tension, blood vessels need an ability to deform and return to form when internal or external pressures are applied. The elastin molecule in the connective tissue layers of blood vessels gives them this property. Elastin, when combined with other extracellular components, helps blood vessels and other tissues, including skin, to return to their original shapes after expansion or deformation. Elastin molecules are disorganized, entangled and crosslinked. When stretched, these fibers straighten out, but will recoil back to normal when force is released on the tissues.
Collagen
Collagen is a stiff, filamentous extracellular protein found in most connective tissues. Collagen filaments are composed of twisted protofilaments, and protofilaments themselves are also twisted. Thus, collagen has ropelike qualities that provide stiffness. In blood vessels, collagen is found in the tunicia externa and provides stiffness to the blood vessel to prevent rupture. Arteries contain blood at higher pressure than veins so they have a thicker layer of collagen than veins.
Blood as a Connective Tissue
Blood is a liquid connective tissue. Connective tissues are defined as tissues with multiple cell types and a large extracellular matrix component. The blood cells, described before, serve different functions and the plasma acts as the extracellular matrix. Blood is a unique, complex fluid with various physical characteristics and functions needed to maintain a stable internal environment in the body. Blood volume is the combination of hematocrit (45 percent) and plasma volume (55 percent). Hematocrit (hct) is the fraction of blood that is composed of red blood cells (RBCs). Besides RBC, leukocytes (white cells) and platelets make up the rest of the formed elements. The process of producing blood cells is called hematopoiesis. Stem cells are nascent cells in the bone marrow that differentiate into various types of formed elements in blood.
Muscle Cells of the Heart
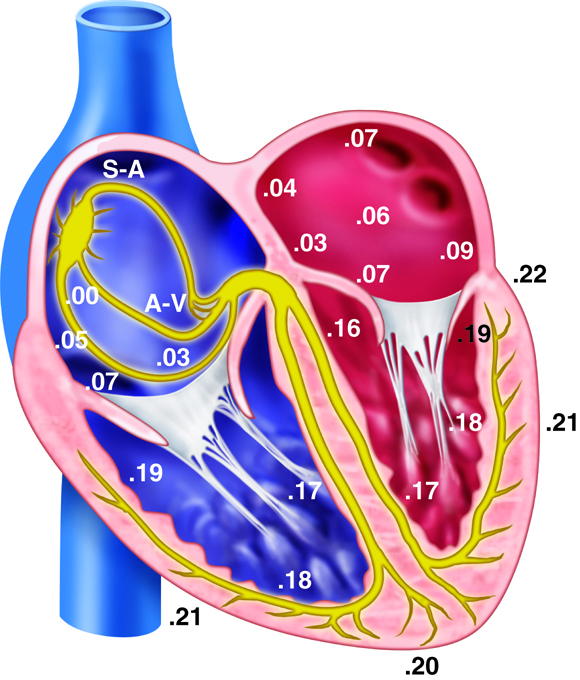
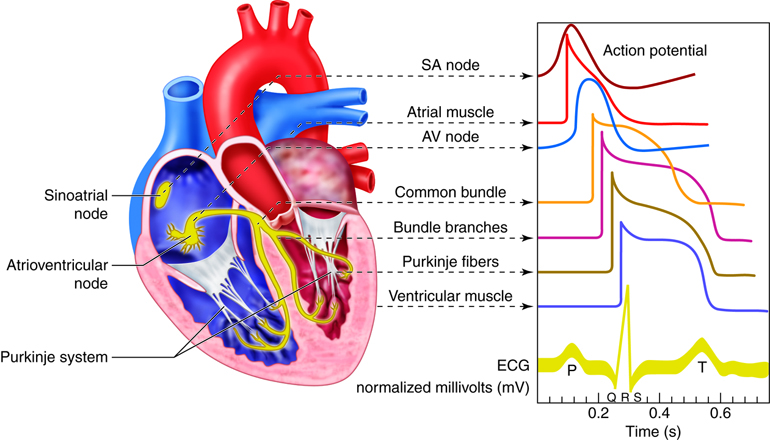
There are three types of cells that comprise the myocardium: the pacemaker, the conducting, and the myocardial cells. The pacemaker cells, also called the nodal cells, are the cells found within the sinoatrial (SA) and the atrioventricular (AV) nodes. These cells spontaneously depolarize, creating the action potentials (or electrical impulses) that start the signal for atrial and ventricular contraction. Specialized conducting cells carry the action potentials at a high rate from the SA to the AV node, and then from the AV node to the rest of the ventricular cells. Some of these specialized conducting cells have been named after the people who described them. For example, once action potentials move through the septum in the proximal part of the AV node, they enter the Bundle of His and then move rapidly through the Purkinje fibers that terminate in the ventricles. Conducting cells are necessary to coordinate contraction by disseminating the electrical signal through the ventricles.
Pacemaker Depolarization
The pacemaker cells in the nodes have the ability to spontaneously depolarize until they reach their threshold value. In this way, they can produce action potentials without the aid of nerves, neurotransmitters or neighboring cells. Each of the action potentials they generate spreads through the heart and results in a contraction. Because the SA node spontaneously depolarizes at a faster rate than the AV node it typically paces the heart, a process called overdrive suppression.
When the threshold voltage is achieved, the action potential is initiated. In skeletal muscle cells and neurons, the rapid depolarization at the beginning of the action potential is due to the opening of voltage-gated sodium channels; however, in cardiac pacemaker cells, these channels do not open. Instead, L-type calcium channels open, allowing calcium to rapidly flow into the cells to generate the upstroke. This dependence on external calcium to generate an action potential instead of sodium is an important distinction between cardiac and skeletal or neuronal cells.
Myocardial Cells
The third type of cell in the heart is the myocardial cell. Comprising 99% of the mass of the heart, these cells are the cardiac muscle cells that contract and carry out the flow generating function of the heart. Without these cells, there would be no contraction.
Cardiac muscle cells are connected together by intercalated discs. These disks physically connect adjacent cells, and include a set of specialized proteins called gap junctions, that can directly pass electrical signals. Because of this, depolarization of one cell can ultimately cause depolarization of all the cells within the heart. This means that cardiac cells act together as a single unit, allowing the heart to function as a whole to contract properly and pump the blood efficiently. In the heart, gap junctions allow ions such as calcium to flow quickly and unimpeded from one cardiac cell to the next. The presence of intercalated disks and gap junctions increase the electrical conductivity of the heart, allowing the action potential to rapidly spread throughout all the cells of the heart.
Heart Conduction
The heart’s ability to generate its own beat is an intrinsic quality. Inside the superior wall of the right atrium a group of specialized cells called the sinoatrial (SA) node spontaneously depolarize, creating a signal that passes to the atria and the ventricles, causing their contraction. Because the SA node repeatedly creates this signal, it is often called the pacemaker of the heart. These action potentials create the rhythmic contraction of the heart that pushes blood through the heart and circulatory system.
If the SA node becomes dysfunctional, the atrioventricular (AV) node, found in the septal wall between the atria and ventricles can take over the pacing of the heart. Its cells also exhibit the property of spontaneous depolarization, but it does so at a slower rate than the SA node; thus the SA node typically “wins” the pacing race by generating action potentials at a higher rate. This is called overdrive suppression.
Because of this intrinsic quality, the heart is said to have automaticity, meaning it is able to self-generate a beat. The heart also has rhythmicity, which means it can generate its own pace of beating. In fact, if a healthy heart is removed from a person or animal, it will continue to beat outside of the body as long as it is put in a medium that supplies it with energy. This occurs as hearts are transported for transplantation.
Action Potentials and Spontaneous Depolarization
Although the heart can intrinsically generate its own rate of spontaneous depolarization (and therefore heart rate), this rate is under significant influence by the autonomic nervous system. The sympathetic and parasympathetic branches of the autonomic nervous system innervate the SA and AV nodes, adjusting their rates of depolarization. The sympathetic nervous system will increase SA node depolarization rates, increasing heart rate. The parasympathetic nervous system has the opposite effect. At rest, the parasympathetic nervous system exhibits greater control of heart rate. If this parasympathetic influence is removed, the heart rate typically increases to about 100 beats per minute. Similarly, the pacemaker cells within the AV node also spontaneously depolarize, but they do so at a rate of about 50 beats per minute.
Electrical Conductance of the Heart
When the cells in the SA node depolarize, the action potential that is initiated is spread throughout the heart. The signal travels through the heart muscle in two ways: (1) from node to node along the conducting cells through the internodal conducting pathway and (2) directly by cell–to-cell spread. The internodal conducting pathway is actually made up of 3 separate paths of conducting cells that connect the SA node with the AV node. Once the signal reaches the AV node, it passes through the septum by conducting through the Bundle of His and into the Purkinje Fibers. From here the signals are distributed throughout the ventricular myocardium in rapid fashion.
Not only do action potentials travel through the internodal conducting cells from node to node, but they also travel directly from cell to cell in the atria and ventricles themselves. Therefore, when cells of the SA node depolarize, the cells surrounding the SA node also depolarize. Because of the gap junctions between the cells, the electrical signal travels quickly throughout all the individual muscle cells of the atria.
Measuring Heart Electricity
The electrocardiogram, also called an ECG or EKG, measures the electrical activity associated with the heart. By placing a minimum of three electrodes on the skin in three locations—for example, the left arm, the right arm, and left leg—the electrical signals that initiate contraction of the atria and ventricles can be recorded. These recordings, or waveforms, are seen on a monitor and are called a tracing. The resultant waveform consists of P, QRS, and T waves. These deflection waves are indicative of the depolarization electrical activity just before the contraction of the atria (P wave), the depolarization electrical activity stimulating ventricular contraction (QRS complex), and repolarization of ventricles prior to relaxation (T wave).
ECG tracings can provide information about the anatomy and placement of the heart in the chest cavity. It provides information about the size of the chambers and the rhythm of contraction. Importantly, ECGs are used to detect changes that have occurred in heart function. Example changes include changes in the rhythm, changes in the conduction pathway of electrical signals, or the development of ischemic areas (areas lacking oxygen) that result from narrowed or occluded coronary arteries.
Changes in the tracing can be used to diagnose many diseases. The P-R interval measures the time from atrial to ventricular contraction. Alterations to the length of this interval can be indicative of damage in AV conductance that is caused by inflammation or medications. The QRS wave shows the electrical activity that initiates ventricular contraction or ventricular systole. Lengthening of this interval can indicate problems with ventricular electrical conduction. During the S-T interval, the ventricles are depolarized and contracting. Normally, the EKG reads zero voltage during the S-T interval, and a value higher or lower than this can indicate that underlying ischemic damage to the heart may have occurred. Ischemic damage results when there is a lack of sufficient oxygen to the heart. The duration of the S-T interval varies with heart rate, decreasing at higher rates.
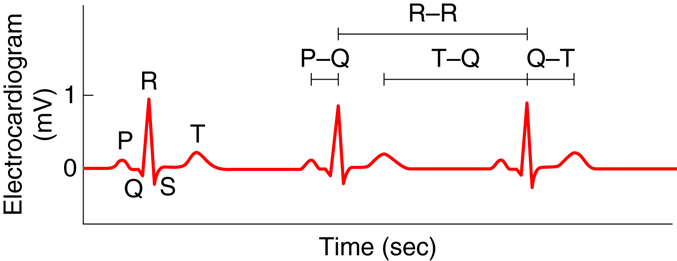
|
Component |
Amplitude (mV) |
Duration (sec) |
|
P wave |
0.2 |
0.10 |
|
QRS complex |
1.0 |
0.08-0.12 |
|
T wave |
0.2-0.3 |
0.16-0.27 |
|
P-Q interval |
N/A |
0.12-0.21 |
|
Q-T interval |
N/A |
0.30-0.43 |
|
T-Q segment |
N/A |
0.55-0.70 |
|
R-R interval |
N/A |
0.85-1.00 |
If the SA node fires rapidly, the person has tachycardia. In one form of tachycardia, atrial tachycardia, the atrial rate of contraction might actually be much higher than the ventricular rate of contraction. This can happen because the AV node can limit the number of action potentials that pass from the atria to the ventricles. On an ECG this would be detected as more P waves than QRS complexes. If the ventricles are continuously firing without a consistently preceding P wave, then the person would have ventricular tachycardia. In this case the QRS complexes also look abnormal due to the pathway of conduction having changed. These are just a few examples of the many abnormalities that can be diagnosed using ECG tracings.
Diseases of the Heart: Arrhythmias
Arrhythmias (or dysrhythmias) are the abnormal rhythms in heart rate or conduction pathway. Some tend to be harmless while others can be fatal. Arrhythmias can range from having an occasional extra beat or a skipped beat to having fibrillation of the atria or ventricles. Arrhythmias can also arise when the heart either beats too slowly (bradycardia) or too quickly (tachycardia).
Arrhythmias are much more serious and deadly when the ventricle beats in an uncontrolled manner. This arrhythmia, called ventricular fibrillation, does not produce a productive heart contraction and will be fatal if not corrected. Atrial fibrillation, on the other hand, is a common arrhythmia arising when multiple cells outside of the SA node spontaneously depolarize at high rates. In this case the atria quiver instead of beat, but because most of the blood in atria move into the ventricles passively due to pressure differences even before the atria contract, it is not nearly as damaging as ventricular fibrillation. People at risk of atrial fibrillation still need to be managed because the condition increases their risk of thromboemboli (blood clots that move to other parts of the body) and stroke. Both atrial and ventricular fibrillation can be diagnosed on an ECG tracing as they will present with either a complete lack of P waves or QRS complexes, respectively.
Cardiac Cycles
The cardiac cycle can be divided into two distinct phases, diastole and systole. During diastole, the muscles are relaxed and the chambers fill passively with blood. Although there is both an atrial and a ventricular diastole, which differ slightly in their timing, the word diastole is commonly used in reference to ventricular diastole. Systole is when the heart muscles contract. Atrial systole helps fill the ventricles while ventricular systole is responsible for pushing the blood into the pulmonary artery and aorta.
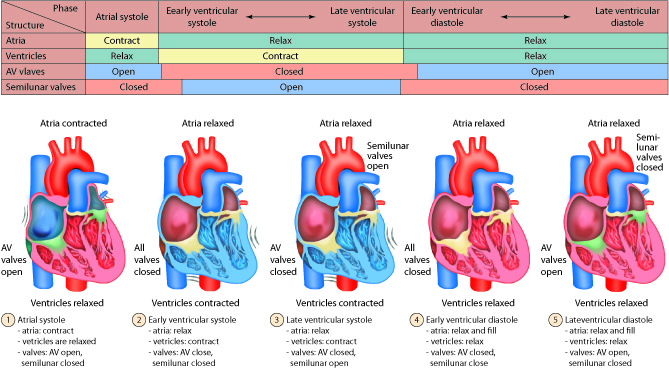
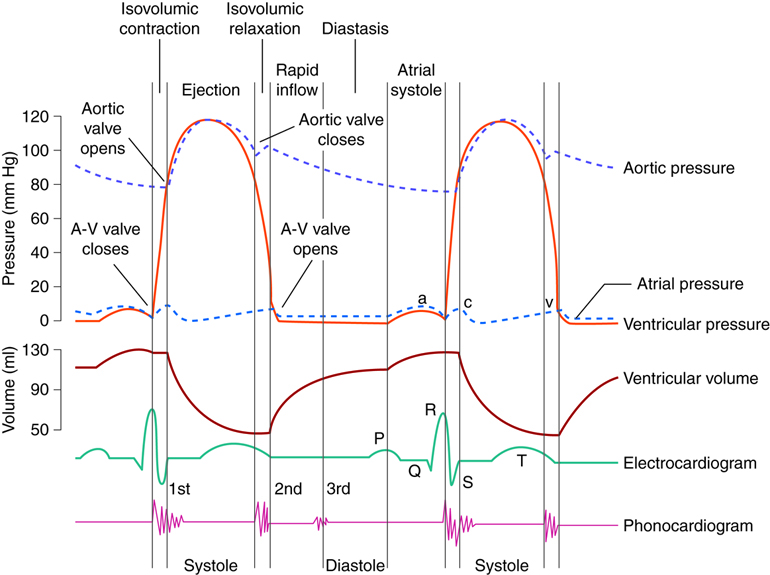
Diastole: Relaxation
During atrial diastole blood enters the left atrium from the pulmonary vein and the right atrium from the inferior and superior vena cava. As ventricular diastole begins, the atria are already well into their diastolic period. The ventricles have just finished contracting, so the ventricular pressure is dropping as relaxation begins. When the pressure within the ventricles drops below the atrial pressure, the AV valves open. Blood passively pours into the atria and flows through the open AV valves into the ventricles. As the blood collects in the ventricle, the pressure builds. In order to continue filling the ventricle , the SA node depolarizes, initiating atrial systole. The contraction of the atrium forces more blood into the ventricle in diastole. Nearly 90% of the blood passively flows into the ventricles while the remaining 10% enter due to atrial systole.
Systole: Contraction
The increasing volume of blood in the ventricles increases the pressure in the ventricles. When this pressure exceeds that in the atria, the AV valves are forced closed. The closing of these AV valves creates the first heart sound (lub). By this time, the atria have finished systole and have entered their diastolic period. There is a delay of about 0.1 sec (100 milliseconds) between the contraction of the atria and the contraction of the ventricles. This delay provides enough time for the atria to complete their contraction before the ventricles begin their turn and prevents the chambers from competing with each other. Imagine if the atria and ventricles contracted all at once. The blood flow would not be as controlled or efficient.
The heart contracts in a repeatable sequence. First the atria contracts (as described above), then the ventricles. This, along with the heart valves, allows the blood to flow in one direction. When the ventricles begin to contract at the onset of systole, both the AV and semilunar valves on either side of the ventricles are closed. This is called isovolumetric contraction. The volume of blood in the ventricles does not change because all the valves are closed, so there is no place for the blood to go. As a result, isovolumetric contraction increases the pressure in the ventricles. When the ventricular pressures exceed the arterial pressures (pulmonary and aortic), the pulmonary and aortic semilunar valves open, ejecting blood into the respective blood vessels. Once the ventricles enter diastole, the ventricular pressures fall. When these pressures fall below that in their respective arteries, the valves close again. This creates the second heart sound (dub). Systole is now over and another diastole has begun; the cycle continues again.
Vascular Tissue
The structural variations between the five major vessel types are due to the relative thickness and composition of three vessel wall layers called tunics. The specific function of each vessel type is dependent on the presence or absence of the three tunics, as well as the extent of the tunic structures. The three tunics, from inside to outside, are the tunica interna, tunica media, and tunica externa.
Tunica Interna
The tunica interna forms the interior lining of blood vessels and is in direct contact with the blood. This layer consists of endothelium, which forms a continuous sheet of cells that lines the lumen of vessels. The endothelial cells are involved in many vessel-related activities. The endothelium is physically supported by the underlying basement membrane. The basement membrane is composed largely of collagen fibers that provide both strength and flexibility. The basement membrane anchors the endothelium to the outermost component of the tunica interna, the internal elastic lamina. The internal elastic lamina is a sheet of elastic fibers with numerous openings that allow movement of solutes between the layers. The internal elastic lamina adheres the tunica interna to the tunica media.
Tunica Media
The tunica media shows the greatest variation among the five vessel types. This layer is composed of smooth muscle and connective tissue layers. In most vessel types this is the thickest tunic. The smooth muscle cells of the tunica media are oriented such that they encircle the lumen of the vessel. These cells regulate the size of the vessel lumen by contracting or relaxing upon stimulus of associated neurons, circulating hormones or local signaling molecules. The flow and distribution of blood, as well as blood pressure, are controlled by the dilation and contraction of blood vessels. The connective tissue of the tunica media is composed of elastic fibers that are interspersed within the muscle cell layers and that also form a separate sheet-like layer, the external elastic lamina, between the tunica media and tunica externa. The elastic fibers allow vessels to expand and recoil without loss of tension.
Tunica Externa
This tunic forms the outermost layer, merging with the surrounding tissues and anchoring the blood vessel in place. The tunica externa is composed of mostly collagen fibers. There are many nerves in the tunica externa and, in larger vessels, a system of vessels that supply the vessel tissues called the vasa vasorum.

