15 Cell Division and Control of Cell Number
Even though we may not feel any changes from day to day, we are constantly repairing and replacing cells within our bodies. It’s estimated that approximately 300 million cells die every minute in our body! These cells must be replaced by identical functional cells for us to survive and maintain homeostasis. The process of cell proliferation or cell division is termed mitosis. Mitosis produces two daughter cells that are identical (clones) to the parent cell.
Cell division is a carefully regulated process. There are “check-points” between phases, in which the cell “self-checks.” Through molecular interactions, the cell makes sure that it is ready to divide; for example, it checks to see that its DNA has not been damaged. If all criteria are met, the cell moves forward through the cell cycle. Normal cells usually divide only a limited number of times, and usually do so when stimulated by certain molecular signals. For example, if you move to higher altitude, your body will produce more red blood cells in order to supply enough oxygen despite the thinner air.
Mitosis is also regulated by genes. Some genes become active to stimulate mitosis and other genes act as the brakes to turn it off (suppressor genes). If our body loses control over mitosis the result would be the production of too many (hyperplasia) abnormal cells and would form a tumor. If the tumor is encapsulated it is termed benign. If not, the mutated cells can spread and it is known as cancer. Cancer cells, on the other hand, have lost the normal cell cycle controls, and therefore can divide indefinitely. (More on this in the below section.)
When a cell is performing its normal day-to-day functions it is in a state termed interphase. When it is time for that cell to replicate, genes become active and stimulate it to begin mitosis. Mitosis is characterized by four phases. Remember if a cell is going to form 2 new identical cells it must first replicate its DNA and organelles. It is during interphase that the DNA is replicated to form 2 identical strands. These strands of identical DNA are termed chromatids and are held together with a centromere.
The major stages of mitosis are:
- Prophase
- Metaphase
- Anaphase
- Telophase
Optional:
For more details on each stage, you can view the below video.
Daily Med Ed. (2015). Mitosis – Made Super Easy – Animation [Video file]. Retrieved from https://youtu.be/ofjyw7ARP1c.
Apoptosis
How does a single fertilized cell develop into something as marvelous and intricate as the human body? Part of the answer is apoptosis, or programmed cell death. For example, the early hand of an embryo is a stubby appendage. As cells between the fingers selectively die off, they sculpt the hand, leaving behind the separate fingers. In adults, apoptosis is one way that tissues maintain their integrity. Cells selectively die off to allow renewal of tissues such as the stomach, lungs, liver and so on. If apoptosis ceases in the tissue’s cells, a tumor can form, perhaps becoming cancerous. Apoptosis is distinguished from necrosis, which is cell death as a result of injury. Much like proliferation, there is a series of tightly regulated events that lead to apoptosis. Apoptosis is a cellular program that is internally determined, and billions of cells die this way every day. However, external trauma, toxins or infection cause a different type of cell death called necrosis. Even though both mechanisms lead to dead cells, apoptosis is a program to maintain cell number whereas necrosis is a consequence of intense cell damage.
Cancer and the Cell Cycle
This section is adapted from Cancer and the Cell Cycle, by Tina B. Jones. Licensed under CC BY-NC-SA 4.0. Retrieved from https://www.oercommons.org/courseware/lesson/57002/overview. For more info about this section, you can visit the OERCommons page for all the works cited.
Cancer is basically a disease of uncontrolled cell division. Its development and progression are usually linked to a series of changes in the activity of cell cycle regulators. For example, inhibitors of the cell cycle keep cells from dividing when conditions aren’t right, so too little activity of these inhibitors can promote cancer. Similarly, positive regulators of cell division can lead to cancer if they are too active. In most cases, these changes in activity are due to mutations in the genes that encode cell cycle regulator proteins.

What’s Wrong with Cancer Cells?
Cancer cells behave differently than normal cells in the body. Many of these differences are related to cell division behavior.
For example, cancer cells can multiply in culture (outside of the body in a dish) without any growth factors, or growth-stimulating protein signals, being added. This is different from normal cells, which need growth factors to grow in culture.
Cancer cells may make their own growth factors, have growth factor pathways that are stuck in the “on” position, or, in the context of the body, even trick neighboring cells into producing growth factors to sustain them1.
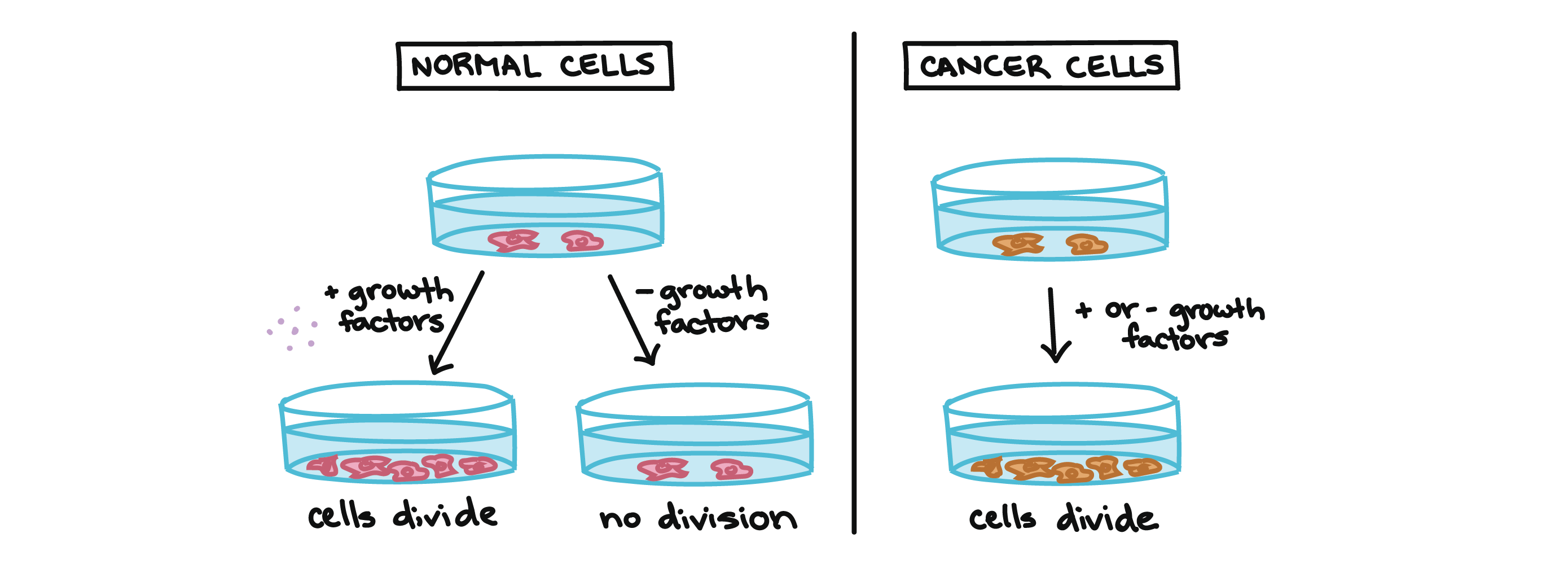
Cancer cells also ignore signals that should cause them to stop dividing. For instance, when normal cells grown in a dish are crowded by neighbors on all sides, they will no longer divide. Cancer cells, in contrast, keep dividing and pile on top of each other in lumpy layers.
The environment in a dish is different from the environment in the human body, but scientists think that the loss of contact inhibition in plate-grown cancer cells reflects the loss of a mechanism that normally maintains tissue balance in the body2.
Another hallmark of cancer cells is their “replicative immortality,” a fancy term for the fact that they can divide many more times than a normal cell of the body. In general, human cells can go through only about 40-60 rounds of division before they lose the capacity to divide, “grow old,” and eventually die3.
Cancer cells are also different from normal cells in other ways that aren’t directly cell cycle-related. These differences help them grow, divide, and form tumors. For instance, cancer cells gain the ability to migrate to other parts of the body, a process called metastasis, and promote growth of new blood vessels, a process called angiogenesis (which gives tumor cells a source of oxygen and nutrients). Cancer cells also fail to undergo programmed cell death, or apoptosis, under conditions when normal cells would (e.g., due to DNA damage). In addition, emerging research shows that cancer cells may undergo metabolic changes that support increased cell growth and division5.
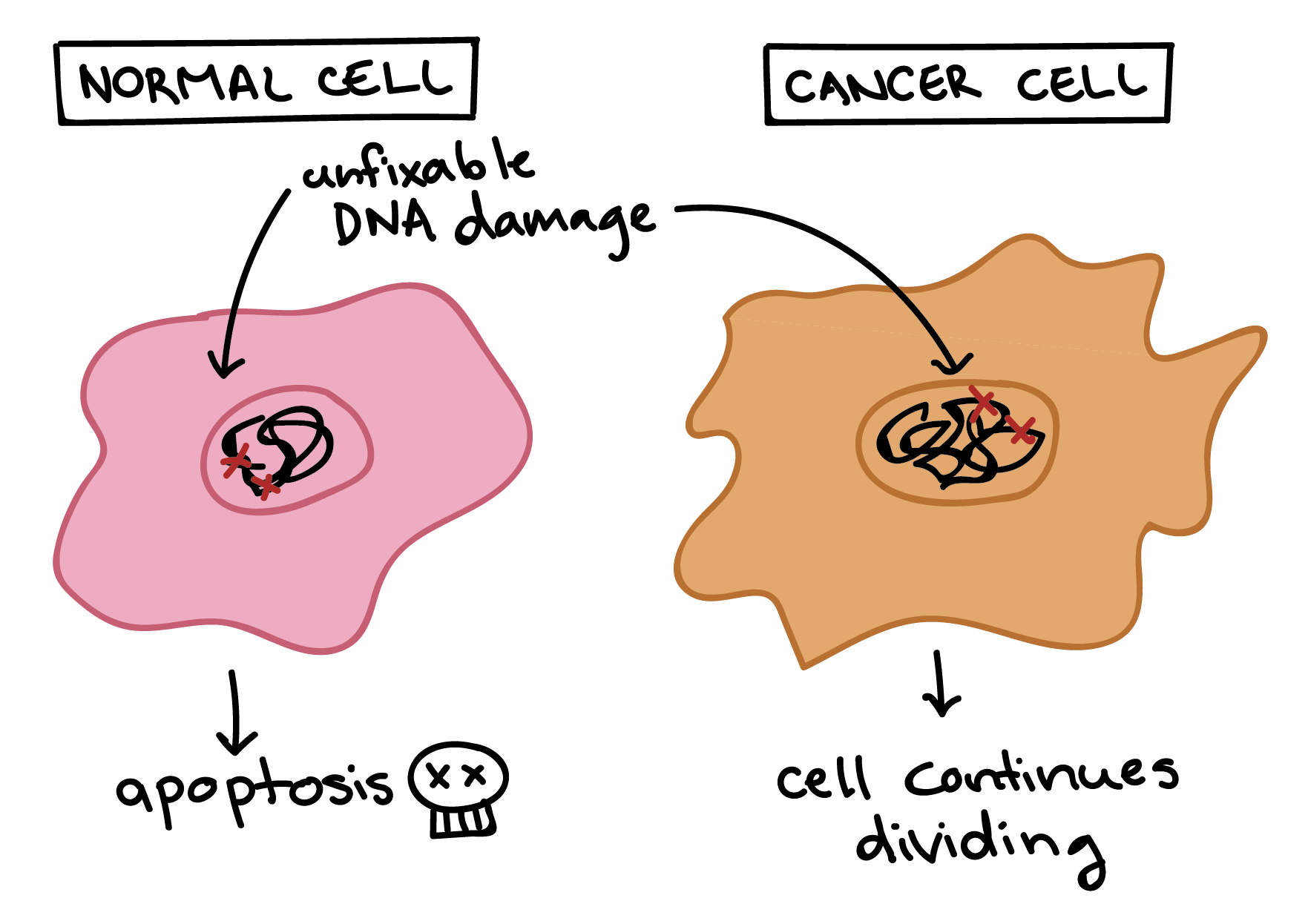
Cells have many different mechanisms to restrict cell division, repair DNA damage, and prevent the development of cancer. Because of this, it’s thought that cancer develops in a multi-step process, in which multiple mechanisms must fail before a critical mass is reached and cells become cancerous. Specifically, most cancers arise as cells acquire a series of mutations (changes in DNA) that make them divide more quickly, escape internal and external controls on division, and avoid programmed cell death6.
How might this process work? In a hypothetical example, a cell might first lose activity of a cell cycle inhibitor, an event that would make the cell’s descendants divide a little more rapidly. It’s unlikely that they would be cancerous, but they might form a benign tumor, a mass of cells that divide too much but don’t have the potential to invade other tissues (metastasize)7.
Over time, a mutation might take place in one of the descendant cells, causing increased activity of a positive cell cycle regulator. The mutation might not cause cancer by itself either, but the offspring of this cell would divide even faster, creating a larger pool of cells in which a third mutation could take place. Eventually, one cell might gain enough mutations to take on the characteristics of a cancer cell and give rise to a malignant tumor, a group of cells that divide excessively and can invade other tissues7.

Optional Video: Incident and Etiology of Cancer – Introduction to the Biology of Cancer #3
Johns Hopkins University. (2019). Incidence and Etiology of Cancer – Introduction to the Biology of Cancer #3. [Video file]. Coursera. Retrieved from https://youtu.be/IiOt3n3m8ak

