42 Cardiovascular Structures and Functions
The Blood
Overview of Blood
The adult human body contains approximately 5 liters of blood (8 percent of the body’s weight), which courses through the heart, arteries, veins, and capillaries. As blood courses through blood vessels it carries out essential functions in the body, such as transporting nutrients, gases, hormones, and heat toward the body’s tissues and carrying waste material, carbon dioxide and excess heat away from these same tissues. The blood also plays an important role in protecting the body against microbial infection and forming clots to protect against blood loss. It takes about 60 seconds for blood to travel completely through the body and return back to the heart.
Blood Properties: color, pH, specific gravity and viscosity
Arterial blood that flows into the aorta from the left ventricle is fully oxygenated so that it can deliver the oxygen necessary for the tissues to make adequate ATP. The combination of oxygen bound to the heme groups in the red blood cells gives this blood a red pigment. Venous blood, which returns to the right side of the heart after having exchanged nutrients in the tissues, appears darker in color because it contains a larger fraction of hemoglobin that is no longer combined with oxygen. In the pulmonary circulation, it is the pulmonary artery blood that is less oxygenated, and therefore darker in color in compared to the brighter red blood in the pulmonary veins.
The heme groups in the red blood cells are part of larger hemoglobin molecules, which are found in high concentration within red blood cells (RBCs). These oxygen carrying cells typically make up 35-45% of blood volume. Together with a small fraction of white blood cells (WBCs) and platelets, they collectively make up the formed elements of the blood. The rest of the blood is composed of a fluid fraction called plasma. Plasma is composed of water and other substances such as proteins, salts, and gases. The pH of blood plasma ranges from 7.35 to 7.45. It has a specific gravity between 1.050 and 1.060 in adults. Specific gravity is a measure of blood density related to pure water (water has a specific gravity of 1.000), so blood is about 5-6% more dense than water. The specific gravity of blood may vary and can be affected by certain diseases that change the concentration of cells within the plasma.
Besides specific gravity, viscosity is another fluid property that may be of importance in blood. Viscosity is the internal friction liquid exhibits when it tries to flow. For example, honey and syrup have a much higher viscosity than water, and they flow slower under similar conditions than water would. The viscosity of pure water is 1.00, whereas the viscosity of blood can be roughly three to five times that because it contains cells and proteins.
Blood helps in the regulation of body temperature. The blood entering the body’s core (the area in which thoracic and abdominal organs are located) is immediately warmed to a temperature of approximately 38°C (100.4°F). Depending on body temperature, this blood may end up traveling to the skin where it can transfer some of this heat to the external environment.
Overview of Plasma
Plasma is the fluid portion of blood with no cells. Serum is the portion of plasma that does not contain any blood-clotting proteins. Plasma consists of mainly water (90 percent). Other substances found in plasma include proteins, respiratory gases (O2 and CO2), electrolytes (sodium, chloride, calcium, and bicarbonate), nutrients, and waste products (e.g., urea and uric acid). The most abundant of the proteins is albumin, which helps retain water in the vascular compartment. The function of plasma is to transport dissolved nutrients and gases, regulate pH, and contribute to fluid and electrolyte balance.
Plasma Proteins
Plasma proteins, which make up approximately 7 to 9 percent of plasma, consist of albumin, globulins, and fibrinogen. These molecules carry out several important functions within the body. Albumin is the smallest, yet it accounts for the majority of these plasma proteins (60 percent). Albumin is synthesized by the liver and functions to maintain colloid osmotic pressure in the blood compartment. This helps prevent the leakage of fluid into the surrounding interstitial spaces, and helps prevent edema (swelling). Three types of globulins make up about 36 percent of the plasma proteins: alpha, beta, and gamma. The alpha and beta globulins transport lipids and metals in blood. Gamma globulins are the antibodies released by immune cells that attack microorganisms and other foreign molecules. Fibrinogen makes up about 4 percent of plasma proteins and is important in the formation of blood clots.
| Protein | Site of Origin | Function | Percentage of Plasma | |
|---|---|---|---|---|
| Albumin | Liver | Maintains colloid osmotic pressure and contributes to the viscosity of blood | 60 | |
| Fibrinogen | Liver | Contributes to blood clotting | 4 | |
| Globulins (Alpha, Beta, Gamma) | 36 | |||
| Alpha | Liver | Transport lipids and fat-soluble vitamins | ||
| Beta | Liver | Transport lipids and other molecules | ||
| Gamma | Lymphocytes | Immunity | ||
Disorders of Blood Plasma: Hypoalbuminemia
Albumin is a transport protein that carries various small molecules, such as bilirubin, metals, hormones, and medications, through blood. Another important function of albumin is to prevent plasma from leaking into tissues. If albumin levels are low, fluid will leak into the tissues, resulting in edema (swelling). Serum albumin levels typically decrease secondary to liver disease (including hepatitis and cirrhosis), loss of albumin in the urine, poor nutrition, or gastrointestinal disorders (celiac, Crohn’s and Whipple’s disease) that interrupt normal nutrient absorption. Levels may be increased with increased concentration of hormones such as steroids, growth hormone or insulin. The levels of albumin can be measured with a serum albumin test; with normal levels between 3.4 and 5.4 g/dL.
A consequence associated with hypoalbuminemia is that medications that are carried by albumin may have an increased concentration of unbound drug in the plasma. It is the unbound fraction that is active, such that even normal doses may seem like an “overdose”. Hypoalbuminemia occurs in acute and chronic inflammatory responses. As a result, a serum albumin plays an important prognostic role among hospitalized patients; as it is correlated with an increase risk of morbidity and mortality. Hypoalbuminemia commonly occurs among older adults who live in long-term care facilities, hospitalized patients with chronic illnesses, and malnourished children.
The Heart
The heart resides just left of the midline of the body in the chest cavity (thorax), and is protected by the rib cage. It is made of two pumps that are connected by a series of tube-like blood vessels that create the circulatory system; the system of arteries, capillaries, and veins that transport the blood to and from the heart. These pumps are separated by a thick wall called the septum. The circulatory system can be divided into two parts, the pulmonary circulation, which connects the heart to the lungs; and the systemic circulation, which connects the heart to the rest of the body.
The heart pumps continuously to provide tissues and organs with needed oxygen and remove cellular waste materials. On average, the heart pumps 70 to 72 beats per minute, every minute of every day. It is able to pump close to the entire volume of blood in your body every minute. This means that on a daily basis, almost 7200 liters of blood are pumped by the heart. This amount increases when you exercise.
Gross Anatomy and Physiology: The Heart
The heart is separated from the lungs inside the thorax by a membranous sack called the parietal pericardium. This sack is continuous with the outermost layer of the heart called the visceral epicardium. Just deep to the pericardium is the myocardium, which is the muscle layer of the heart. It comprises 99% of the mass of the heart. The endocardium is the innermost layer of the heart tissue that lines the heart chambers and is continuous with the endothelium lining blood vessels.
The heart is made of two pumps, each containing an atrium and a ventricle that is separated by a thick wall called a septum. The heart pumps blood in one direction through the circulatory system to provide oxygen to organs and to remove metabolic waste from the tissues. Each heart beat forces blood through the circulatory system and back to the heart. Gas exchange, which involves releasing carbon dioxide and acquiring oxygen, occurs within capillaries of the lungs.
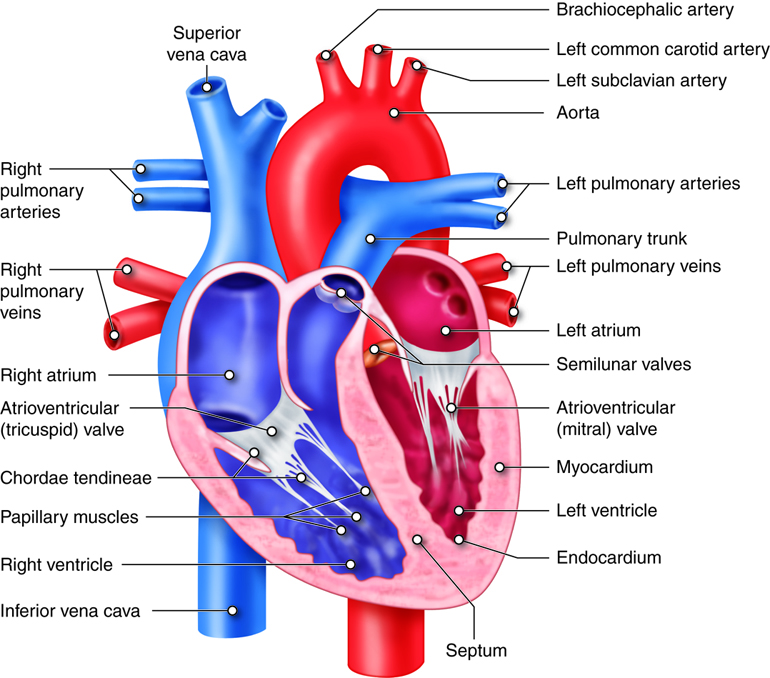
Chambers of the Heart
Blood enters the right side of the heart by streaming into the right atrium, the chamber of the heart that receives blood from the superior vena cava, inferior vena cava, and the coronary sinus. From the right atrium, it flows into the right ventricle. Blood then exits the heart and enters pulmonary circulation, traveling through the pulmonary artery (that carries blood from the heart to the lungs) to the lungs where gases are exchanged. Freshly re-oxygenated blood returns to the left atrium traveling through the pulmonary vein (that carries blood from the lungs to the heart). From the left atrium, it flows into the left ventricle before exiting the heart into the aorta to enter the systemic circulation to be transported throughout the body. This oxygen-rich blood flows through the arteries to the organs where oxygen is delivered and metabolic waste is removed. From the organs, oxygen-poor (more specifically, less oxygenated) blood returns to the heart, carried by the veins, and enters the right atrium again.
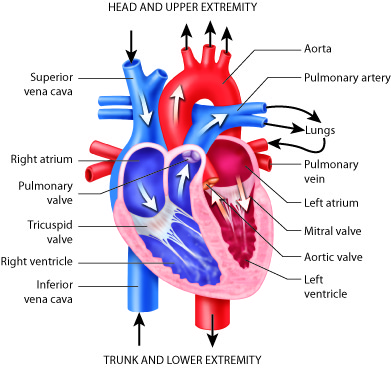
While these muscular pumps work together in the heart, they are not equal in the size of their musculature. The left side contains thicker muscles than the right side. Why? The right side has to generate less pressure in order to pump the blood through the short circulatory pathway of the lungs. This does not require as much pressure as pumping to the systemic circulation, like the left ventricle has to do.
Diseases of the Heart: Atrial Septal Defects
Have you ever heard of having a hole in the heart? A small percentage of people have just that, a hole in the wall (septum) that separates the right and left atria. Medically, this is known as an atrial septal defect. This defect is a congenital disease, meaning it is present at birth. When a fetus is still developing, the septum between the right and left atrium is not fully formed. The atria are connected together by a hole, called the foramen ovale, between the two chambers. This opening allows blood in the fetal heart to be shunted from the right atrium into the left atrium. This shunted blood does not get pumped into the pulmonary artery, as this is not necessary in the fetus. Instead, the fetus receives oxygen (and nutrients) from the placenta via the umbilical cord. When delivery occurs and the newborn takes his or her first breath, the lungs inflate. This changes the pressure distribution in the heart and causes a flap of tissue to close the foramen ovale, which then grows into a completed septum. When the foramen ovale does not close properly, or if there are similar congenital defects, the child is left with an atrial septal defect or, in other words, a hole in his or her heart.
Whether or not this problem needs to be corrected is mainly dependent on the size of the hole. This is because some blood from the left atrium will move through the atrial septal defect into the right atrium, causing it to recirculate through the pulmonarycirculation. If too much blood is shunted back through the lungs it can cause pulmonary hypertension and ultimately heart failure.
Most of the time, symptoms are very mild or not even noticed. Sometimes, symptoms do not develop until much later in life. Atrial septal defects can be treated with surgical closure of the defect.
Valves of the Heart
As mentioned earlier, blood flows through the heart in one direction. What prevents blood from flowing in the opposite direction? The answer is the heart’s valves. These valves maintain unidirectional flow by preventing backward (retrograde) flow when the pressure gradients change during the heart cycle. These valves are found in matched pairs in two locations of the heart. The first set, the atrioventricular (AV) valves, separate the atria from the ventricles. The second, the semilunar valves, are located between the ventricles and the arteries they feed.
Following the path of blood flow through heart, the first valve is the tricuspid valve found separating the right atrium from the right ventricle. This AV valve is so named because it has three flaps: the anterior cusp, the medial cusp, and the posterior cusp. On the ventricle side of the valve are long muscular cords, called chordate tendinae, that extend from the inferior side of each cusp. The chordate tendinae attach to each cusp on one end and to the papillary muscle, which is connected to the inner wall of the ventricle, on the other end. These muscles prevent the valves from inverting back into the atria during ventricular contraction.
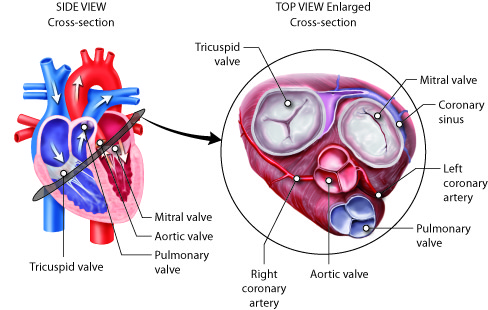
Most of the blood flows passively into the atria, through the tricuspid valve, and into the right ventricle. Atrial contractions then push most of the remaining atrial blood into the ventricle. As the right ventricle fills, the pressure inside it increases. This increased pressure, coupled with the right atria moving into its relaxation phase, causes the ventricular pressures to be greater than atrial pressures. This causes the tricuspid AV valves to close. In this closed position, the chordae tendinae that are attached to the ventricular side of the valves are fully extended and taut, like a stretched bungee cord. The chordae tendinae prevent the higher pressure in the ventricle from forcing the valve to open back into the atria, thus preventing the backflow of blood through the tricuspid valve.
As the blood flows from the right ventricle into the pulmonary artery, it passes through the pulmonary semilunar valve (pulmonary valve). Opposite to the tricuspid, the pulmonary semilunar valve remains closed when the ventricle is filling and opens during ventricular contraction. Again, this is because the valves work by opening when the pressure is higher on their proximal side and close when the pressure is higher on their distal side.
On its return from the lungs, blood enters the left atrium. As the blood passes from the left atrium into the left ventricle, it passes through the bicuspid valve, often referred to as the mitral valve. This valve is similar in structure and function to the tricuspid, but only contains two cusps, the anterior and posterior cusps. It, too, is open when the pressure in the atria exceeds the ventricle and it, too, has chordae tendinae and papillary muscles that prevent its inversion upon ventricular contraction. The release of blood from the left ventricle into the aorta is regulated by the aortic semilunar valve, which is most commonly referred to as the aortic valve. It is similar in structure and function to the pulmonary semilunar valve. Because they regulate the flow of blood through the heart and prevent its backflow, these valves are indispensable for the proper function of the heart.
Diseases of the Heart: Ruptured Chordae Tendinae
The chordae tendinae perform an important function by keeping the cusps of the atrioventricular valves tethered to the interior wall of the ventricles. Without these tendons, and their associated papillary muscles, the valves could become inverted when the pressure in the ventricle increases, causing the blood to rush back into the atria.
Although this happens infrequently, the chordae tendinae can rip or rupture. When this happens, the cusps of the tricuspid or mitral valves are no longer properly tethered to the wall of the ventricle. As the blood presses back against the valves during ventricular contraction, the valves may not remain closed. If many of these connections are ruptured or if the thicker ones are affected, the valves can open in the reverse direction, and blood can backflow into the atria. This results in increased pressure in the atria and, if the backflow is significant enough, congestive heart failure could develop.
Rupture of the chordae tendinae occurs when a patient suffers from a myocardial infarction (heart attack), heart valve infection, or from trauma to the chest. Damage can range from minor to major depending on the number of chordae affected. It is diagnosed by hearing a murmur, or a characteristic whooshing noise, upon auscultation (listening to heart sounds with a stethoscope or other device) of the heart. Ruptured chordate tendinae, and their associated conditions, commonly lead to heart failure.
Rupture of the chordae tendinae attached to the mitral valve is most common, although rupture of those bound to the tricuspid valve can also occur. Rupture of the mitral valve chordae tendinae can result in a rapid increase in left atrial pressure. This can subsequently increase the pressure in the pulmonary circulation, ultimately producing pulmonary edema; which is retention of fluid in the lungs. An echocardiogram can provide a more detailed look into the condition of the heart and the degree of damage to the chordae tendinae.
Pericardium, Myocardium and Endocardium
The heart is protected by the ribcage in the chest cavity. It is enclosed in a sack that provides increased protection. This sack, or pericardium, is a fluid-filled, (although the space is very small, so the volume of fluid is very low) membranous structure that separates the heart from the lungs within the chest. It is made up of the visceral pericardium, which is actually the outer epicardial layer of the heart, and the parietal pericardium. At times, the pericardium can become inflamed, which is a condition called pericarditis.
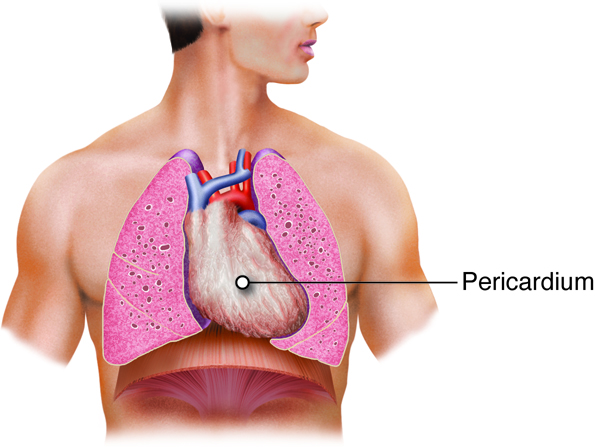
The heart is made of three layers, the endocardium, the myocardium, and the epicardium. The endocardium is the innermost layer of the heart that forms a barrier between the muscle layers of the heart and blood. The myocardium is the thickest of the three layers of heart tissue. It is the cardiac muscle layer that contracts and generates force to pump the blood. The outermost layer of the heart is the epicardium. It is a thin layer of cells that covers the outside of the heart and forms the visceral layer of the pericardium. The epicardium protects the heart within the thorax.
Diseases of the Heart: Cardiac Tamponade
Inflammation of the pericardium can result in cardiac tamponade. When the pericardium gets infected fluids or blood can accumulate between the layers of the pericardium. This increases the pressure that is exerted on the outside of the heart and prevents the heart from filling appropriately during diastole. If the heart does not fill normally, the stroke volume decreases. The body can try to compensate with an increased heart rate and force of contraction (both effects of the sympathetic nervous system), but if this is not enough, the person will go into heart failure.
Blood Flow Through the Heart
Blood circulates in a closed system. It flows from the heart, through the lungs, back to the heart, and then to the rest of the body. The blood always returns to the heart. The amount of blood that circulates and the rate blood circulates is controlled by the pumping of the heart. The system is similar to having a rubber ball filled with water and a tube connected on both sides of it. When the ball is squeezed, the water enters the tube on one side. When the ball is relaxed, water is drawn into it through the other side. Squeeze again, and more water is ejected into the tube. Relax again, and water is drawn into the ball. The valves between the opening of the tube and the ball, which only open one way, control the entry of water into the tube and prevent its tendency to move backwards.
Blood continuously flows in a circle. Oxygen-poor blood enters the lungs where carbon dioxide, a waste product of cells, is exchanged for oxygen. This oxygen-rich blood returns to the heart by way of the pulmonary veins. When it arrives from the lungs, it enters the left atrium. Some of the blood flows passively through the bicuspid (or mitral) valve into the left ventricle. When the left atrium contracts, the remaining blood in the left atrium is pushed through the bicuspid valve into the powerful left ventricle.
After the left ventricle contracts, the blood exits through the aortic valve into the aorta to be circulated throughout the body. The first section of the aorta is the ascending aorta, which runs only a short way before becoming the aortic arch at the apex of an inverted U, from which vessels branch to supply the head, neck and arms. After the aortic arch turns downward it becomes the descending aorta. The thoracic aorta supplies blood to the thorax above the diaphragm and the abdominal aorta supplies blood to the region below the diaphragm. Arteries that branch off of the aorta continue to branch into smaller and smaller arterioles and eventually form the capillaries within the organ. It is within the capillaries that the gas exchange occurs. In the organs, oxygen is provided and carbon dioxide is removed. This is the opposite of gas exchange in the lungs where carbon dioxide is removed and oxygen is provided.
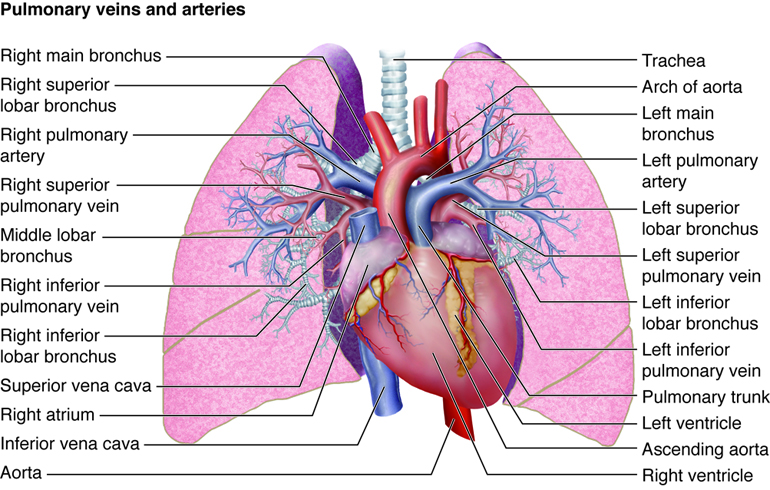
Once exchange has occurred, oxygen-poor blood exits the capillaries, flowing into venules, or small veins. These venules combine to form veins, which eventually return the blood to the right atrium. The blood from the head, neck and arms returns to the right atrium through the superior vena cava, while the blood from the lower body returns through the inferior vena cava. The right atrium contracts and blood passes through the tricuspid valve into the right ventricle. From the right ventricle, the blood is ejected through the pulmonary semilunar valve into the pulmonary artery to travel to the lung arterioles and capillaries. Oxygen-rich blood flows into the venules and back to the heart through the pulmonary vein and is ready to enter the left atrium again and circulate around the body again.
Venous Return
The volume of blood in the systemic veins that is flowing back to the heart is called venous return. Blood returns to the heart through veins because of the difference in pressure between venules (approximately 16 mmHg, on average) and the right ventricle (0 mmHg). Any pressure increase in the right ventricle or right atrium lessens venous return. A leaky (“incompetent”) tricuspid valve raises right atrial pressure, because it allows blood to flow backward (“regurgitate”) when the ventricles contract. The resulting reduction in venous return is accompanied by an accumulation of blood in veins of the systemic circulation.
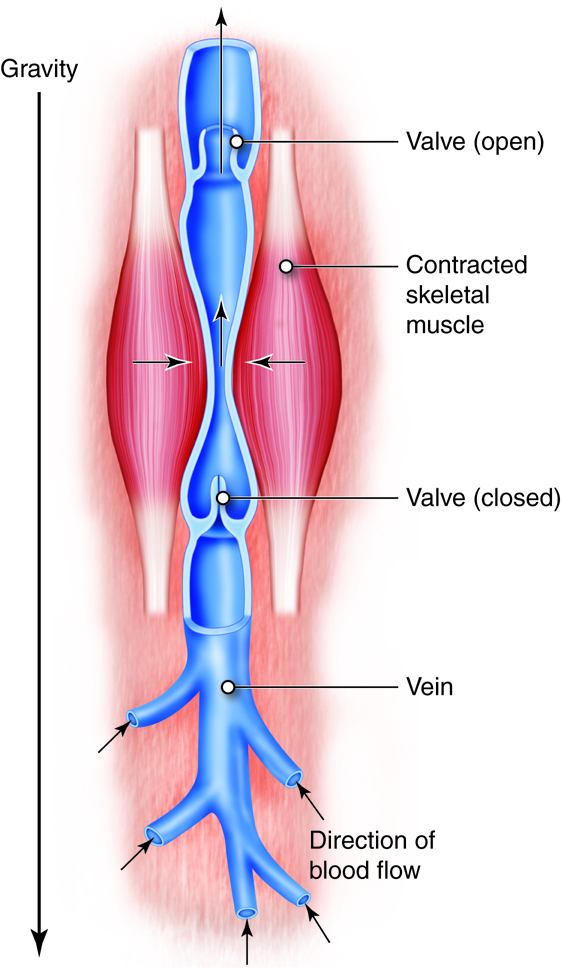
In the upright individual, return of venous blood from the lower body is particularly challenged. This is because the flow of blood has to overcome the effects of gravity as well as the normal resistance of the vessels. Therefore, two additional factors help force blood toward the heart: the skeletal muscle pump and the respiratory pump. Both mechanisms rely on venous valves to operate.
The skeletal muscle pump uses muscle contractions in the legs to force venous blood upwards toward the heart. The peripheral veins in the legs have one-way valves that route blood away from the legs and toward the heart. When standing still, these valves prevent the backflow of blood that might otherwise occur due to gravitational pull. When muscles of the legs contract, they tend to “squeeze” the veins of the legs; propelling blood. Because of the one-way valves, blood will only move toward the heart, helping maintain venous return. Lack of use of this skeletal muscle pump contributes to the lower leg edema common in people who are bedridden or confined to a wheelchair. When flow in the legs is not maintained, the venous system there becomes congested. This leads to increased capillary hydrostatic pressure and resultant edema.
Sequential compression and decompression of veins also drives the respiratory pump, which operates on abdominal and thoracic veins. When you inhale, downward movement of the diaphragm reduces the pressure in the thoracic cavity and increases the pressure in the abdominal cavity. The resulting compression of abdominal veins pushes blood into the decompressed thoracic veins and then into the right atrium. When you exhale, the pressures reverse, and the one-way valves in the veins prevent blood from flowing back to the abdominal veins from the thoracic veins.
The Fetal Circulation
The path that blood takes through the fetal circulatory system and heart differs somewhat from that in the postpartum state. In particular, a majority of the blood passes through three shunts, bypassing the liver and lungs that do not require as much blood in the fetus as they do after birth. These shunts include the ductus venosus, which bypasses the liver, and two shunts that bypass the lungs; the foramen ovale and the ductus arteriosus.
The lungs are also not functional in the fetus, as the placenta carries out the functions of gas exchange. In fact, the lungs are deflated, with the alveoli collapsed. This condition also narrows the vessels of the pulmonary vasculature, creating high resistance to blood flow. To keep the workload on the right heart from becoming too high in the fetus, most of the blood bypasses the lungs. This occurs via two shunts. The first is an opening between the right and left atria called the foramen ovale. Because the pressures are higher in the right heart than the left heart in the fetus (due to the high pulmonary resistance), blood moves from the right atrium to the left atrium through this shunt. The remaining blood moves into the right ventricle where it is pumped into the pulmonary artery. But even from here most blood will not go through the remainder of the pulmonary circulation. Instead, it travels from the pulmonary artery to the aorta through the ductus arteriosus.
Soon after birth the lungs inflate, greatly reducing the resistance through pulmonary circulation and lowering the pressures on the right side of the heart. This briefly reverses flow through the foramen ovale, pushing two flaps of tissue over the opening, closing it. These flaps grow into the atrial septum, leaving a slight depression called the fossa ovalis. The decreased pressure in the pulmonary artery also triggers the ductus arteriosus to collapse, converting it into the connective ligamentum arteriosum over the next three months or so. The ductus venosus become a connective tissue remnant, called the ligamentum venosum, found on the inferior surface of the liver.
Vessels of the Heart
Force generating cells of the heart require energy to beat constantly and the conductive cells require energy to maintain electrical potentials. So the heart, itself, requires a large supply of blood. It is the role of the coronary circulation to deliver this blood to the heart. In this circulation, the coronary arteries deliver the oxygen-rich blood to the myocardium while the cardiac veins are responsible for returning the blood back to the right atrium.
Coronary arteries on the surface of the heart are called epicardial coronary arteries. These arteries are commonly affected by atherosclerosis and can become blocked, causing angina or heart attack. The branches of the coronary arteries penetrate the myocardium and work their way inward, ending up as subendocardial arteries.
The right and left coronary arteries originate from the aortic sinuses, which exit from the aorta right after it leaves it heart. The posterior interventricular branch of the right coronary artery supplies the right and left ventricles. The right ventricle also receives blood from the second branch of the right coronary artery, the marginal branch. The left coronary artery has two branches: the anterior interventricular branch (left anterior descending branch), which supplies both ventricles, and the circumflex branch, which supplies the left ventricle and left atrium.
Aging and the Heart: Valvular Stenosis
If any of the four valves within the heart become stiff, the valves are unlikely to open to the fullest extent. Blood flow is impeded and the pressure in at least one of the heart chambers increases.
Mitral valve stenosis increases the stiffness of the mitral valve that separates the left atrium from the left ventricle. This results in increased pressure in the left atrium, which backs up into the pulmonary circulation, causing edema there. If the atria can’t compensate enough to maintain flow through the narrowed mitral valve, heart failure will develop. People with untreated mitral stenosis typically develop atrial hypertrophy in an effort to generate enough atrial pressure to maintain this flow. Similar results and compensatory mechanisms occur in tricuspid valve stenosis.
Aortic valve stenosis occurs when the aorta valve stiffens. This increases the pressure in the left ventricle and increases the stress developed in the wall of the left ventricle during ejection. The stroke volume is reduced as the left ventricle has to contract more forcefully to eject the blood into the aorta. This causes left ventricular hypertrophy, although this is not always enough to maintain flow.
Heart Calculations
Cardiodynamics and Cardiac Output
Cardiac output is one measure of the heart’s efficiency. It is the amount of blood pumped per minute. Heart rate fluctuations are one way for the body to adjust the cardiac output so that it can appropriately meet the demands of the body. When resting, the body doesn’t have as high of a demand for oxygen because the muscles and organs are at a lowered metabolic rate compared to when they are in a state of exertion. Therefore, the heart rate is low. Similarly, if the body is actively exercising, the demand for oxygen and the need to get rid of carbon dioxide are both quite high. The heart rate, and therefore the cardiac output, increases accordingly.
Control of Cardiac Output Through a Changing Stroke Volume
Cardiac output is the product of heart rate (beats per minute) and stroke volume (volume of pumped blood per beat). Although the heart has the intrinsic ability to generate a consistent heart rate, we have already learned that the autonomic nervous system exerts significant control over this variable. Similarly, there are factors that affect the stroke volume of the heart. The amount of blood that flows into the heart is termed preload. Besides preload, the stroke volume of the heart can also be affected by myocardial contractility, and, under certain conditions, afterload, which is the pressure the ventricle needs to develop to eject blood.
We have already discussed heart rate. It is the pace the heart beats or the number of beats per minute. It is easy to see how an increase or decrease in the number of beats can affect the overall cardiac output. If the heart beats more often, more blood will be pumped through the heart. If the cardiac output is a function of heart rate times the stroke volume, then as the heart rate increases, so does the cardiac output. At least this is true up to a point. At very high heart rates the heart may not have time to fill appropriately during diastole, which can actually decrease cardiac output. This is one of the risks of tachyarrhythmias (tachycardias).
Circulatory Pathways: Blood Vessels
The human cardiovascular system consists of two separate circulatory routes, the pulmonary circuit and the systemic circuit. In each circuit, several levels of blood vessels work in sequence to receive blood from the heart, deliver it to the appropriate tissues and cells, and then return blood to the heart. The sole function of the pulmonary circuit is to carry blood to the alveoli of the lungs so that gases can be exchanged. The function of the systemic circuit is to deliver oxygenated blood and nutrients to the entire body, with the exception of the lungs. The pump for the pulmonary circuit is the right side of the heart, which receives oxygen-poor- blood from the systemic circulation. The pump for the systemic circuit is the left side of the heart, which also collects the oxygen-rich blood returning from the lungs.
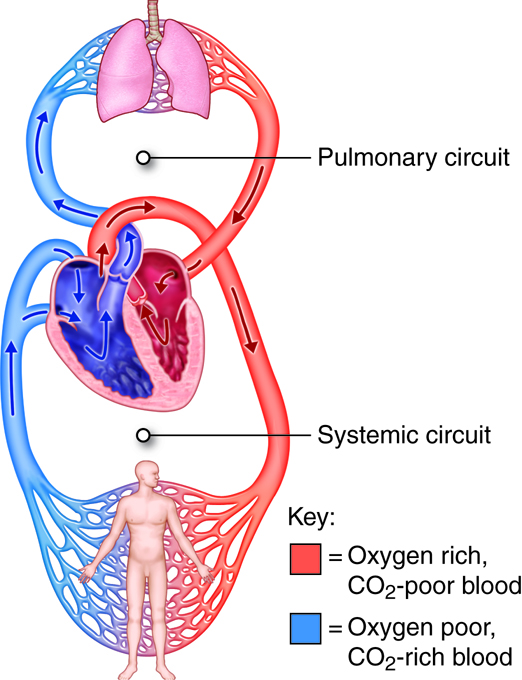
There are two important differences between the systemic circulation and pulmonary circulation. The first is that blood in the systemic circulation must be pumped farther than blood in the pulmonary circulation. The second is that, in pulmonary arteries, the diameter is larger, the walls are thinner, and the tissue is more elastic than in systemic arteries. Because of the characteristics of pulmonary arteries, there is very low resistance to blood flow in the pulmonary circuit, and thus pressures do not need to be very high to move blood through the lungs. In the right ventricle, the peak systolic pressure is typically one-fifth that in the left ventricle.
In general, the arteries and arterioles of the systemic circuit deliver blood, rich with oxygen and nutrients, to capillary networks interspersed within the organs and tissues. The systemic venules and veins drain the capillary networks and return the blood, high in carbon dioxide and waste products, to the right side of the heart. In contrast, the arteries and arterioles of the pulmonary circuit deliver oxygen-poor blood to the capillaries surrounding the alveoli (singular: alveolus) of the lungs. After carbon dioxide is exchanged for oxygen, the pulmonary venules and veins return oxygen-rich blood to the left side of the heart. The arteries, arterioles, capillaries, venules, and veins are categorized by wall structure, location in the circuit, and function. Blood in systemic arteries is bright red in color. Blood in capillaries is dark red, because it has lost some oxygen and gained carbon dioxide.
Gross Anatomy and Physiology: Blood Vessels
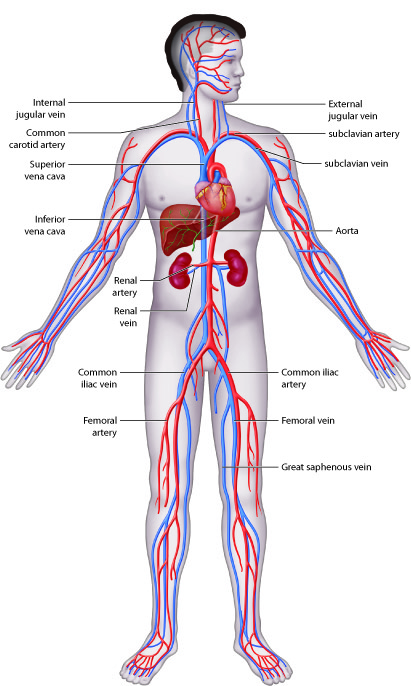
Systemic circulation begins when blood from the left ventricle is pumped into the aorta. The aorta and its branches deliver blood to all body tissues except for those in the lungs. Venous blood from the systemic circuit is returned to the right atrium by way of the venae cavae and the coronary sinus. The superior vena cava receives blood from veins that drain the head, neck, chest, and upper limbs. The inferior vena cava receives blood from veins that drain the abdomen, pelvis, and lower limbs. The coronary sinus drains the myocardium.
The aorta is divided into the ascending aorta, the arch of the aorta, the thoracic aorta, and the abdominal aorta. Branches off of the ascending aorta supply the atria and ventricles of the heart. The aortic arch has three major branches: the brachiocephalic trunk, left common carotid artery, and left subclavian artery. These arteries and their branches supply the head, neck, brain, and upper limbs. Branches of the thoracic aorta and abdominal aorta supply structures of the thorax and abdomen, respectively. Branches of the abdominal aorta also supply the lower limbs.
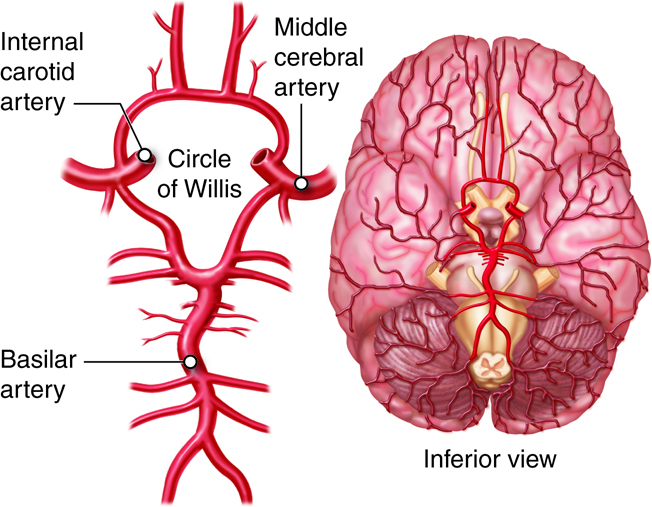
The arterial supply of the brain includes an arrangement of arteries called the arterial circle (circle of Willis). These vessels provide collateral pathways that ensure uninterrupted blood supply to the brain if some arteries are blocked. The venous drainage of the brain is unique in that blood drains first into large chambers called dural sinuses instead of veins.
The arterial supply of the thorax and abdomen includes both visceral and parietal branches. The visceral branches perfuse the organs, and the parietal branches perfuse the wall structures. Venous drainage of the digestive organs includes the hepatic portal circulation, which sends blood to the liver for nutrient processing and toxin removal before it is returned to the systemic circulation.
The arterial supply of the upper limbs is primarily a continuing sequence of vessels rather than branches of other vessels. The subclavian artery continues as the axillary artery, which continues as the brachial artery.
The pelvis and lower limbs are supplied by the internal and external iliac arteries, respectively. These arteries are branches of the right and left common iliac arteries, which are the terminal branches of the abdominal aorta.
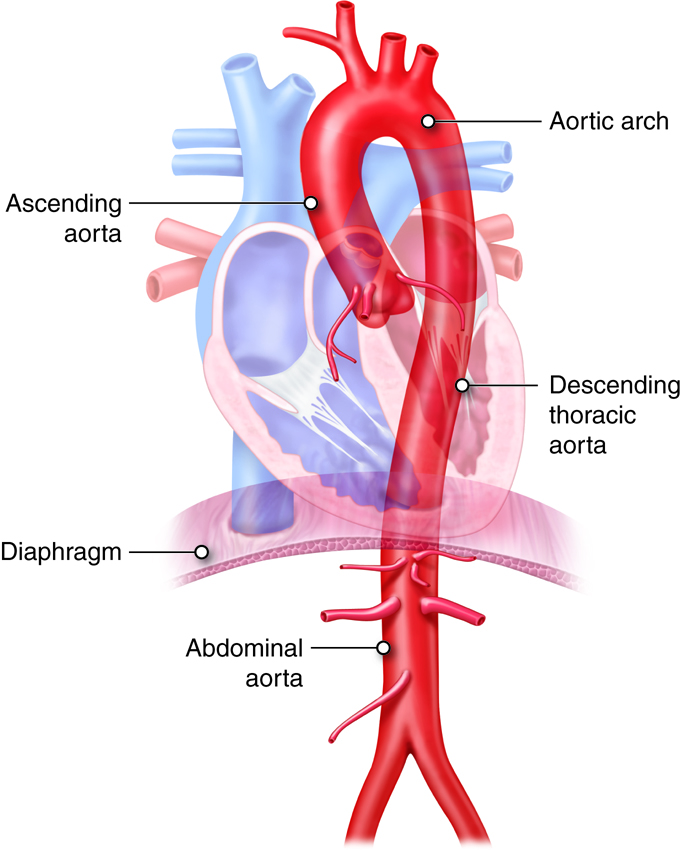
The Aorta and Its Major Branches
The aorta is the biggest artery in the body, with a diameter of approximately 3 cm (1 in.). All systemic arteries branch off the aorta. The aorta has four major segments: ascending aorta, arch of the aorta (or aortic arch), thoracic aorta, and abdominal aorta. Arteries that branch off each section of the aorta divide into smaller arteries that supply different organs. These arteries then divide into arterioles within the organs, and finally into capillaries that supply all systemic tissues except the alveoli of the lungs.
The ascending aorta, which is approximately 5 cm (2 in.) long, is the first section of the aorta. It begins at the aortic valve, at the upper part of the base of the left ventricle. It has three dilations called aortic sinuses. The right and left coronary arteries originate from the right and left aortic sinuses.
The ascending aorta curves to the left and becomes the arch of the aorta, which is about 4–5 cm (2 in.) long. It runs downward and ends in front of the border between the fourth and fifth thoracic vertebrae. The arch of the aorta gives rise to three major branches: the brachiocephalic trunk, left common carotid artery, and left subclavian artery.
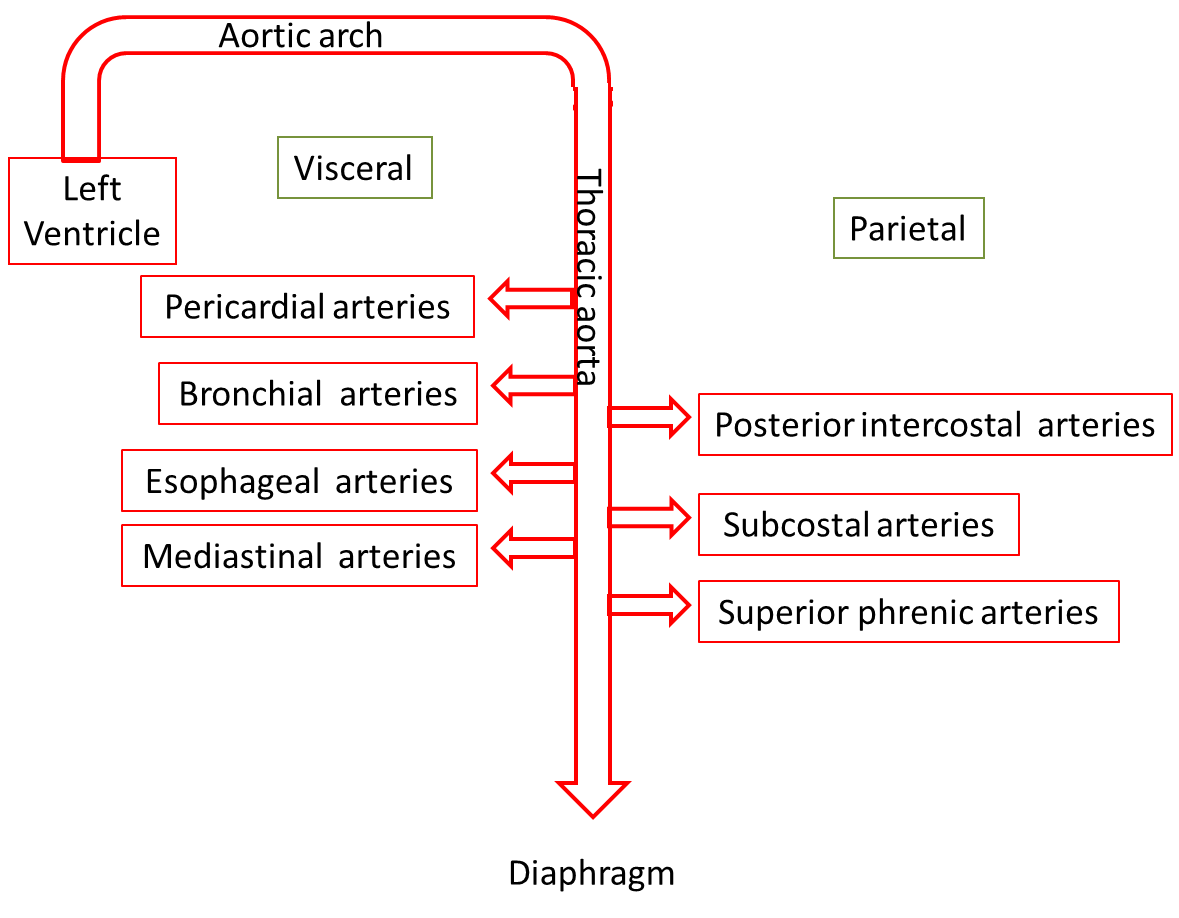
As the aorta continues its descent, it passes through the aortic hiatus, an opening in the diaphragm. The part of the aorta between the arch of the aorta and the diaphragm is called the thoracic aorta. It is approximately 20 cm (8 in.) long and starts at the border between the fourth and fifth thoracic vertebrae. The thoracic aorta gives rise to a number of small arteries. The visceral branches supply the viscera, and the parietal branches supply the body wall structures of the thorax.
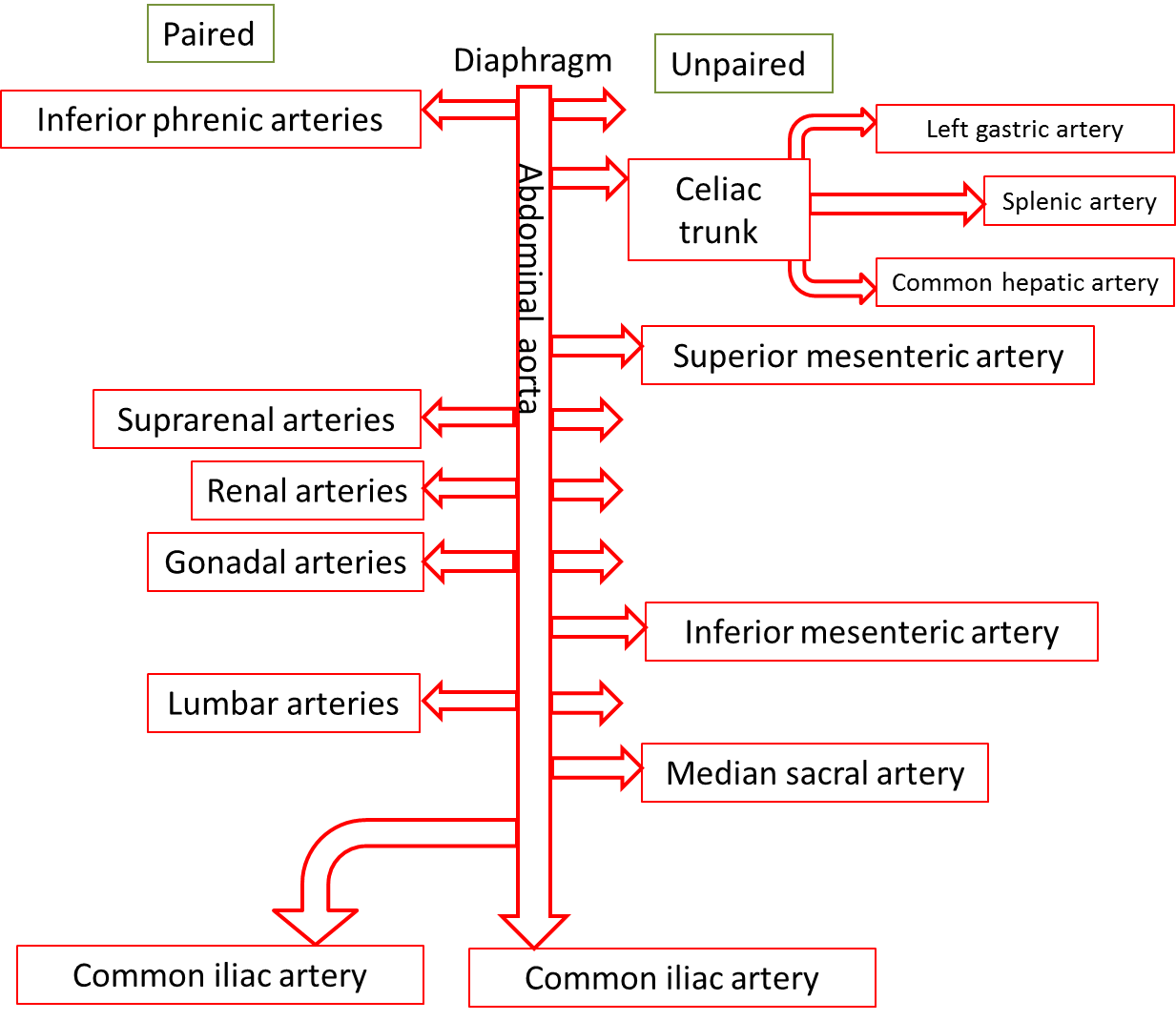
The abdominal aorta is the part of the aorta between the diaphragm and its bifurcation (division into two branches) into the two common iliac arteries at the level of the fourth lumbar vertebra. The abdominal aorta is about 13 cm (5.1 in.) long. Like the thoracic aorta, the abdominal aorta gives off visceral and parietal branches.
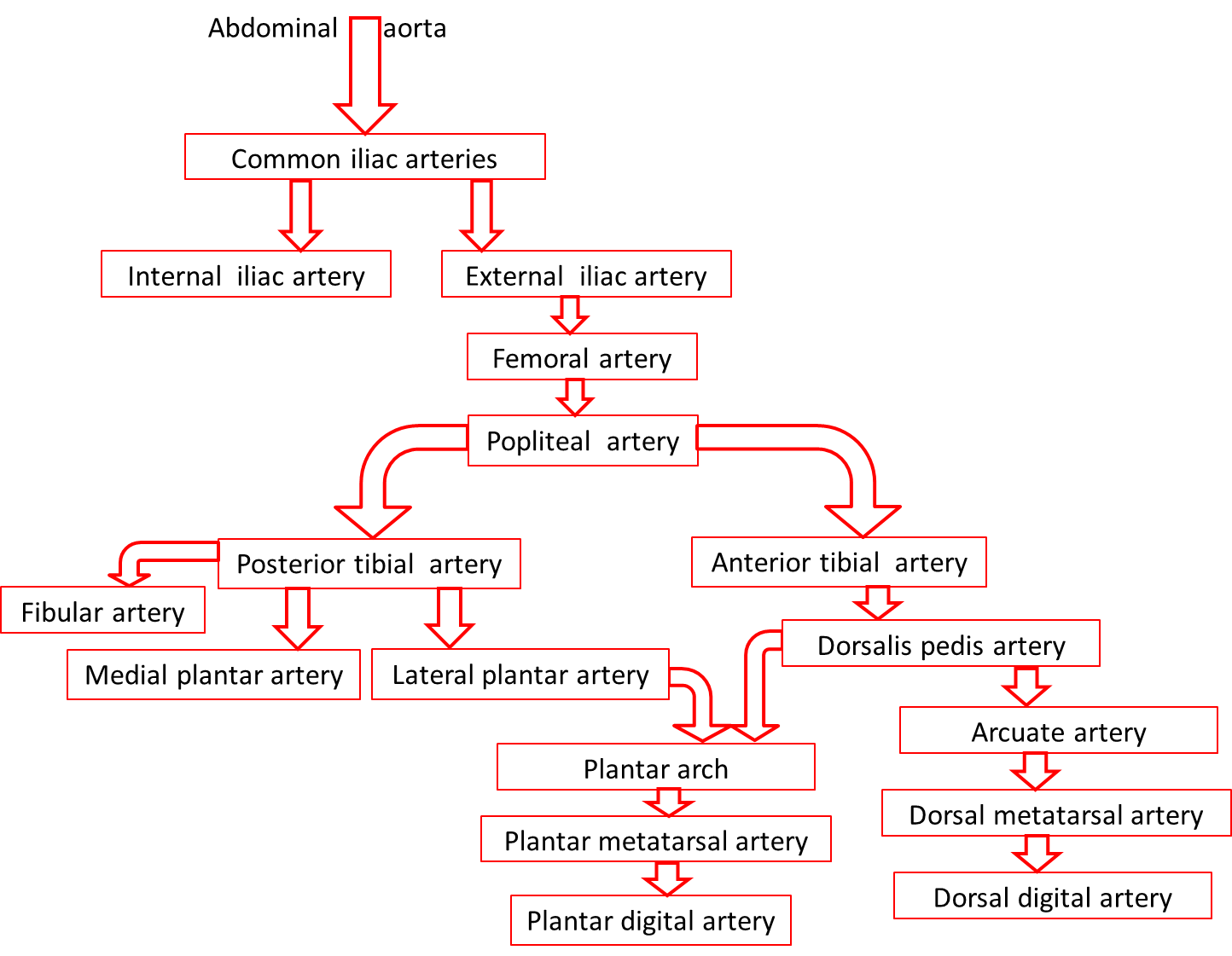
Arteries
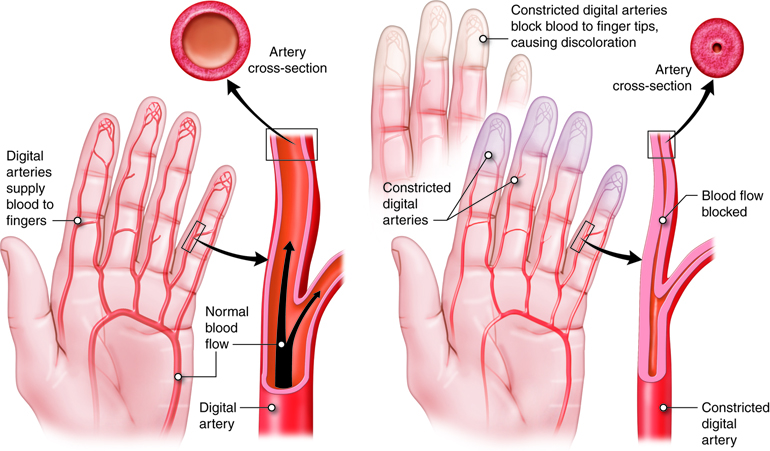
Arteries are vessels that carry blood away from the heart. The tunica media is the thickest tunic in arteries, although all three layers are present. The smooth muscle cells in the tunica media of arteries are innervated by fibers of the autonomic nervous system. When stimulated, the muscle cells contract and cause vasoconstriction, a decrease in the diameter of the blood vessel lumen. Relaxation of the muscle cells causes vasodilation, an increase in diameter of the lumen. Damage to an artery also triggers vasoconstriction to help prevent blood loss. As arteries move blood away from the heart, they branch into smaller and smaller vessels.
Artery Types
Arteries are typically classified by location, but they can also be described by their function.
Elastic Arteries
As the name indicates, elastic arteries have vast capacity for expansion and retraction. These properties are due to the internal and external elastic laminae and extensive elastic fiber sheets interspersed within the muscle cells. The arteries with the largest diameters in the body are elastic arteries. The elastic arteries that receive blood from the heart, such as the aorta, the common carotids, and the pulmonary trunk, play a critical role in regulating the pulsing blood that is ejected from the heart. These vessels temporarily store blood by expanding and then slowly recoiling. This action propels the blood forward at a more consistent rate than is delivered during systole and also helps to maintain blood pressure during diastole. The elastic arteries are also called conducting arteries because they direct blood to the next smallest diameter vessels, the muscular arteries.
Muscular Arteries
Compared to elastic arteries, muscular arteries have a smaller diameter and a higher percent of muscle cells to elastic fibers in the tunica media. Muscular arteries are typically medium-sized vessels with a very thick tunica media. The extensive smooth muscle cells in muscular arteries allow for greater vasoconstriction and vasodilation. This property of muscular arteries contributes to the regulation of blood flow. Muscular arteries are also called distributing arteries because they direct blood into the various organs, continuing to branch into smaller and smaller diameter vessels. Some examples of muscular arteries are the brachial, radial, ulnar, and coronary arteries.
Arteries of the Head and Neck
The head and neck are supplied by the paired common carotid arteries and three paired branches of the right and left subclavian arteries: the vertebral arteries, thyrocervical trunks, and costocervical trunks.
Branches of the common carotid arteries supply blood to the head and brain. The right common carotid originates from the brachiocephalic trunk. The left common carotid is the second branch of the aortic arch. At the “Adam’s apple,” the common carotids divide into the external and internal carotid arteries. The external carotid artery supplies most external head structures, except the orbits. The internal carotid artery supplies the orbits and more than 80 percent of the cerebrum. The internal carotid arteries have a carotid sinus with baroreceptors that help regulate BP. Pressure on the neck near the carotid sinuses can cause unconsciousness, because it simulates high blood pressure, eliciting a reflex vasodilation and interfering with blood flow to the brain.
Branches of the vertebral arteries supply blood to the neck structures and the spinal cord. They originate from the subclavian arteries at the base of the neck and unite in the brain stem to form the basilar artery. Costocervical trunks arise from the subclavian arteries and supply blood to the deep neck muscles and some intercostal muscles of superior rib cage.
Arteries of the Thorax
The wall of the thorax is supplied by a collection of arteries, some of which originate directly from the thoracic aorta and others from branches of the subclavian artery. Two bronchial arteries on the left and one on the right deliver systemic blood to the lung structures, including the visceral pleura, esophagus, and bronchi of lungs. Blood is also delivered to the esophagus by the four or five esophageal arteries. Numerous small mediastinal arteries deliver blood to the posterior mediastinal structures. Nine pairs of posterior intercostal arteries wrap around the rib cage before anastomosing anteriorly with the anterior intercostal arteries.
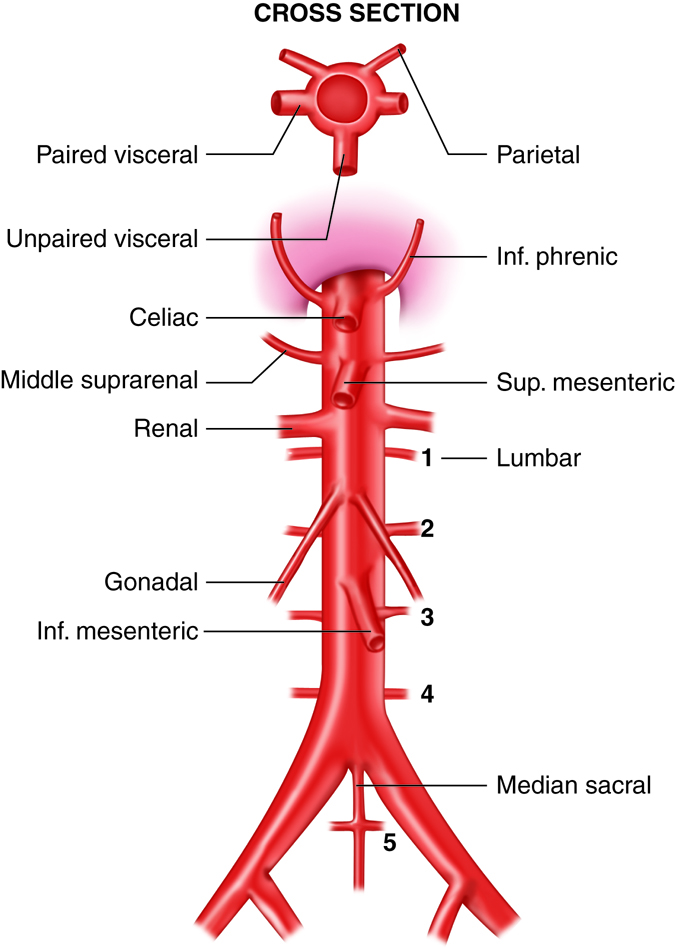
Arteries of the Abdomen
Arteries that supply the abdominal organs and abdominal wall structures originate from the abdominal aorta. About half of resting cardiac output flows through these vessels. The abdominal arteries are all in pairs, except for the superior and inferior mesenteric arteries, the celiac trunk, and the median sacral artery.

Arteries of the Upper Limbs
The three major arteries that supply the upper limbs are a continuing sequence of vessels, not branches of other vessels. The axillary artery is a continuation of the subclavian artery, and the brachial artery is a continuation of the axillary artery. The right and left subclavian arteries send branches off to the neck. Both arteries then run laterally between the clavicle and first rib into the axilla, where they become the axillary arteries.
Arteries of the Pelvis and Lower Limbs
The right and left common iliac arteries are the terminal branches of the abdominal aorta. Each of these arteries branches into internal and external iliac arteries. The internal iliac arteries and their branches supply the pelvis, while the external iliac arteries and their branches perfuse the lower limbs.
Arterioles, Metarterioles, Capillaries, and Venules
Arterioles
The smallest of the vessels that carry blood away from the heart, thearterioles, direct blood into the capillary networks. Both thetunica interna and tunica externa of arterioles are thin and the tunica media consists of only one or two layers of muscle cells that encircle the vessel. However, these arterioles are particularly important in regulating the amount of flow delivered to the tissues that they feed. These arterioles have a rich supply of sympathetic nerve fibers. When they receive a high number of signals from the sympathetic nervous system (i.e. a high degree of sympathetic tone), the arterioles constrict, increasing resistance to blood flow. If the signals from the sympathetic nervous system decrease (i.e. decreased sympathetic tone), the arterioles dilate, reducing the resistance to flow. Arterioles can also adjust their diameters in response to hormonal signals (such as angiotensin II) and local signaling molecules (such as prostaglandins). Fine adjustments to the diameter of the arteriole lumen have a direct effect on the flow of blood into the capillary network supplied by that particular arteriole. Arterioles are numerous and do not have individual names as the elastic and muscular arteries do.
Metarterioles
Metarterioles are short vessels that connect arterioles to thecapillary networks. Metarterioles do not have a true tunica media. Instead, at the metarteriole-capillary junctions a single smooth muscle cell forms a ring around the metarteriole. Each encircling muscle cell acts as a precapillary sphincter, regulating the flow of blood into thecapillaries that branch from the metarteriole. In response to stimuli, the muscle cell encircling the metarteriole contracts, reducing the size of the lumen. This prevents the flow of blood into the capillaries fed by that metarteriole. If most or all of the precapillary sphincters associated with a capillary network contract simultaneously, blood is moved directly from the arterial to the venous system through the metarteriole. In this situation, the metarteriole is acting as a thoroughfare channel, and the entire capillary network is bypassed. Because each metarteriole regulates blood flow into a specific number of capillaries, blood flow through any tissue is finely controlled. Blood delivery to a particular tissue can be quickly increased, decreased, or even temporarily halted in order to respond to the current metabolic activity of the tissues they supply.
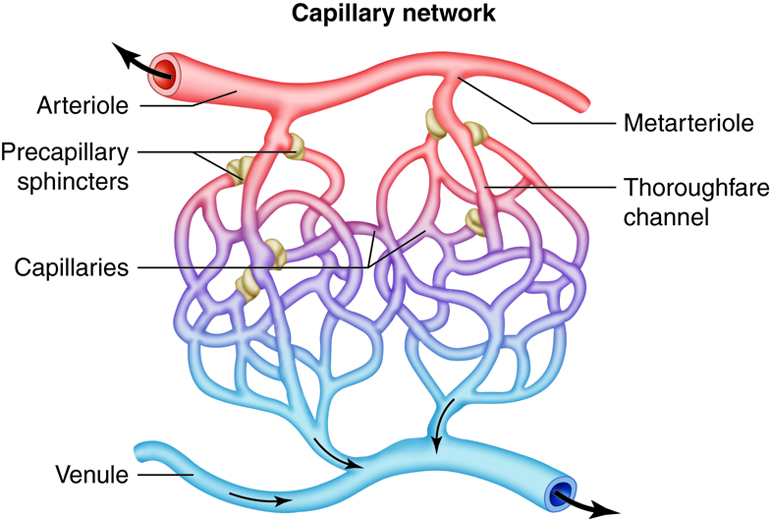
Capillaries
The smallest of all the vessels, the capillaries form extensive networks throughout the entire body. Capillary networks form the connections between the arterial and venous systems. The complexity of each capillary network varies in response to the metabolic needs of the tissues served. Tissues with high oxygen requirements, such as skeletal muscle, have 8 or 10 capillaries branching from each metarteriole. Tissues with lower needs, such as the intestinal tract have 2 or 3 capillaries from each metarteriole. There are even some tissues, such as nail beds, with metabolic needs so low that the ratio of metarteriole to capillary is one to one.
In addition to capillary network design, capillary wall construction further facilitates the rapid exchange of gases and solutes. Capillary walls consist of only a tunica interna. Decreasing the distance that substances must travel increases the diffusion rate. The artery and arteriole wall thickness prevents diffusion of gases and solutes from the blood until it arrives at the target tissue. In some tissues the demand for oxygen and nutrients is so high that even the thin barrier of the tunica interna prevents adequate diffusion rates. To accommodate the specific needs of each tissue and organ, there are three different types of capillaries, based on wall structure. Following the exchange of gases and solute, capillary networks drain into postcapillary venules.
Capillaries are classified by the structure and arrangement of their endothelial cells and the underlying basement membrane. The three types, continuous capillary, fenestrated capillary, and sinusoid capillary, each have a characteristic structure that dictates the level of permeability. Continuous capillaries have the lowest permeability rate of the three types and are common in muscle, nervous, and connective tissues. Fenestrated capillaries allow for faster solute exchange but still exclude larger molecules from passing through. The endothelial cells of fenestrated capillaries have numerous fenestrae (“windows”). They are common in areas that require rapid absorption or filtration such as the small intestines, the kidneys and the choroid plexuses of the brain. Sinusoid capillaries are limited to areas where blood cells move in and out of the bloodstream. The endothelial cells are widely separated and contain large pores without membrane coverings. Sinusoid capillaries are found where blood cells are formed, such as red bone marrow, and where large proteins enter the bloodstream, such as the liver.
Venules
At the venous end of a capillary network, the capillaries that branch from asingle metarteriole reunite and empty into a venule. As blood moves into the venules, and thus the venous system, the return trip to the heart begins. The walls of venules and veins are much thinner than those of arteries and arterioles. Without blood within, arteries retain their shape but veins collapse. The postcapillary venules collect blood from the capillary networks. These smallest and most porous of the venules are involved in solute and gas exchange in the tissues along with the capillary networks. Postcapillary venules are also the site of white blood cell exit from the bloodstream in response to inflammation or infection. In a reversal of the branching of arteries into smaller and smaller vessels, venules coalesce to form larger and larger vessels, gaining vessel wall components and thickness as they enlarge.
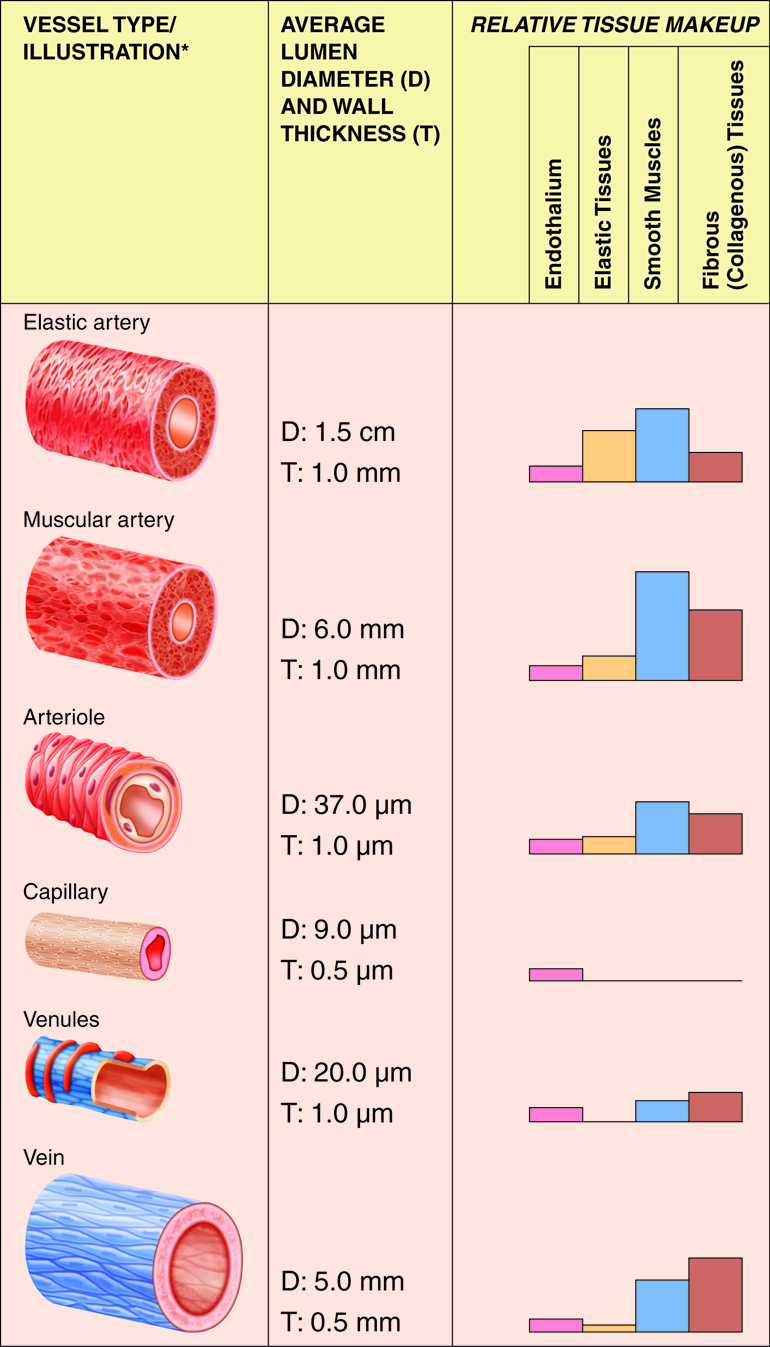
Veins
Although all three tunics are present in veins, the tunica interna and tunica media are quite thin, and both the internal and external elastic laminae are absent, or very thin. These features render the veins capable of great expansion to hold the variable volume of blood passing through them. At any given time, there is three times as much blood volume in the venous system than there is in the arterial system. However, veins are not designed to handle the high blood pressure commonly found in arteries. Due to the relatively large lumen and thin walls, veins will appear flattened in a micrograph since the vessel collapses when it is not filled with blood.
Blood pressure in the venous system is much lower than blood pressure in the arterial system (10 mm Hg compared to 90–100 mm Hg). Therefore, the flow of blood back to the heart from the capillary networks, called venous return, cannot depend on pressure alone. One feature of veins that augments venous return is the presence of venous valves within the vessels, most commonly in the limbs. The valves of veins are formed by extensions of the tunica interna that form flaps into the vessel lumen. These valves close when blood in a vein tries to move backward, away from the heart. The closed valve forms a barrier to the backward flow of blood.
Along with the differences in wall structure, the venous system has some unique features. One of these is the presence of venous sinuses, large-diameter veins with extremely thin walls that contain no smooth muscle. Support is instead provided by the dense connective tissue surrounding the vessel. Venous sinuses are collection points for oxygen-poor blood that is headed back to the heart. Examples are the coronary sinus of the cardiac venous system and the dural sinuses in the brain. In addition, veins are more numerous than arteries. Often two veins will accompany a single artery. Between the veins are connecting channels, called anastomoses. Anastomoses are most commonly found in the limbs, and the veins that form them are not always associated with arteries. In the upper and lower limbs, superficial veins are present where arteries are not. Anastomoses connect the superficial veins to the deeper veins which are accompanied by arteries. Also unique to the venous system are portal veins. Portal veins drain one capillary network, travel to another organ or tissue, and empty into another capillary network. The hepatic portal vein carries nutrient-rich blood from the gastrointestinal tract and spleen to the liver.
Veins that Return Blood to the Heart
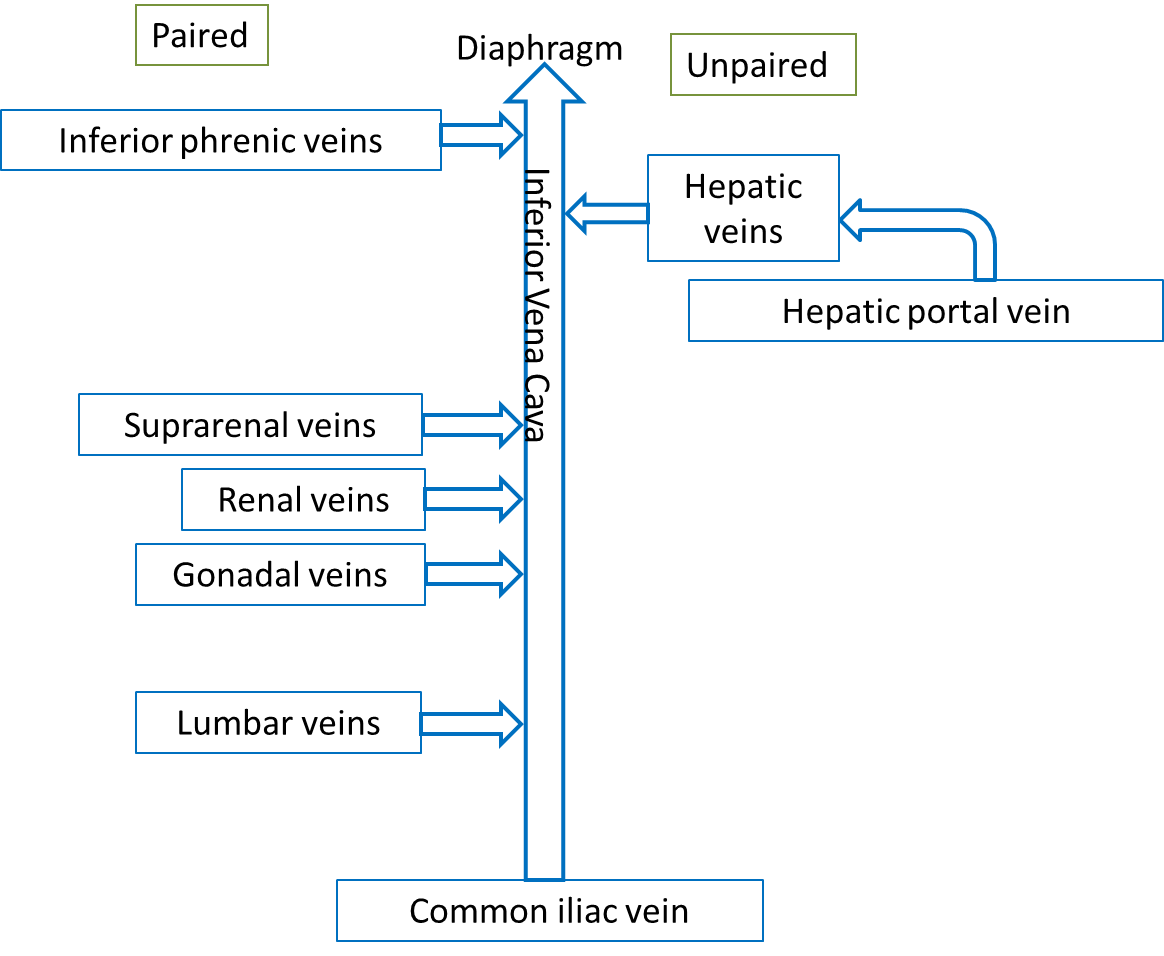
Venous blood from the systemic circuit is returned to the right atrium by way of the venae cavae and the coronary sinus. The superior vena cava receives veins that drain the head, neck, chest, and upper limbs. The inferior vena cava receives veins that drain the abdomen, pelvis, and lower limbs. The coronary sinus drains the myocardium.
The majority of blood that returns to the heart flows through the superior and inferior venae cavae. The names of these veins are derived from the location of their tributaries. The superior vena cava collects blood from veins located superior to (above) the diaphragm, with the exception of the wall of the heart and the alveoli of the lungs. The superior vena cava, which delivers blood to the right atrium, is formed by the merger of the right and left brachiocephalic veins. Each of these veins, in turn, is formed by the union of the internal jugular vein and subclavian vein on the right or left side of the body. The inferior vena cava, which is the largest-diameter blood vessel in the body, collects blood from veins located inferior to (below) the diaphragm and delivers it to the right atrium. This vein is located directly to the right of the abdominal aorta. Its distal end is created by the merger of the paired common iliac veins. Blood that drains from the myocardium of the heart empties into cardiac veins and returns to the right atrium via the coronary sinus.
Veins of the Head and Neck
The three major vein pairs that drain blood from the head and neck are the internal and external jugular veins and the vertebral veins. The courses and interconnections of these veins are significantly different than the arterial system that supplies the head. Most of the veins that drain the brain empty into the dural sinuses. These large, interconnected chambers lie between the dura mater layers.
The internal jugular veins originate from the dural venous sinuses and drain most blood from the brain. They collect blood from deep face and neck vein branches of the facial and superficial temporal veins. The right and left internal jugular veins merge with the subclavian veins to form the right and left brachiocephalic veins.
The external jugular veins are superficial veins (right and left) that empty into the subclavian veins. When venous pressure increases (e.g., during coughing or conditions such as congestive heart failure), these veins bulge conspicuously at the side of the neck. They drain the scalp, as well as superficial and deep regions of the face. Deep structures of the neck are also drained by the vertebral veins. Right and left vertebral veins run through transverse foramina of the first six cervical vertebrae before exiting at the sixth cervical vertebrae and entering the brachiocephalic veins at the base of the neck.
Veins of the Thorax
The brachiocephalic vein of the thorax carries blood that is on its way back from the head, neck and upper limbs, as well as collects blood from the mammary glands and from the first two or three intercostals spaces. The right and left brachiocephalic veins unite to form the superior vena cava. The left brachiocephalic vein is longer than the right because of the superior vena cava’s location to the right of the body’s midline.
Most blood from the thoracic wall and tissues empties into an amalgamation of veins called the azygos system. This system offers a collateral pathway for draining areas served by the inferior vena cava, including the abdominal wall. A variety of anastomoses connect the azygos system with the inferior vena cava. The veins of the azygos system border the vertebral column laterally. Large veins draining the lower limbs and abdomen empty into the azygos system. The azygos system can drain venous blood from the lower body if the inferior vena cava or hepatic portal veins are damaged.
Veins of the Abdomen
Blood from the abdominal walls and abdominal (and pelvic) organs returns to the heart via the inferior vena cava. Blood from the digestive organs empties into veins that drain into the hepatic portal vein. This common vessel carries the venous blood into the liver. The hepatic veins then carry the blood from the liver to the vena cava. This hepatic portal system includes two sets of capillary beds located between the arterial supply and the ultimate venous drainage. Capillary beds in the stomach and intestines empty into tributaries of the hepatic portal vein, which carries the blood to the capillary bed in the liver. The hepatic portal system also includes a variety of tributary vessels from the stomach and pancreas, including the superior and inferior mesenteric veins and the splenic vein. The superior mesenteric vein collects blood from all of the small intestine, the ascending and transverse colons of the large intestine, and the stomach. The splenic vein drains the spleen and portions of the pancreas and stomach. This vein unites with the superior mesenteric vein to create the hepatic portal vein. Blood from the distal parts of the large intestine and rectum drains into the inferior mesenteric vein, which unites with the splenic vein immediately prior to its merger with the superior mesenteric vein to form the hepatic portal vein.
Veins of the Upper Limbs
The deep veins of the upper limbs have similar courses and names as their companion arteries. The superficial veins are not paired with arteries, but provide an anastomotic network for draining the limbs. The large superficial veins in the upper limbs are visible just below the skin.
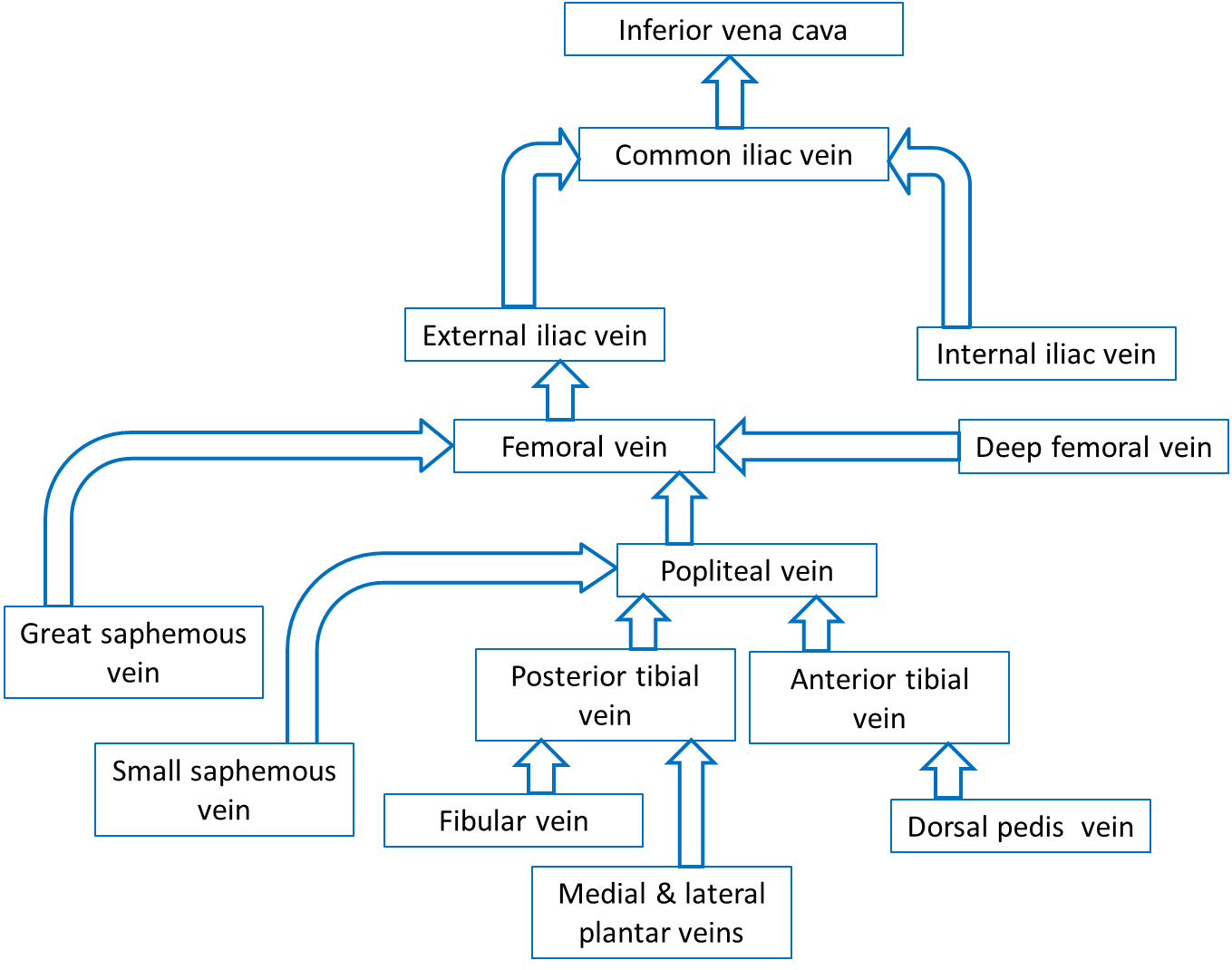
Veins of the Pelvis and Lower Limbs
Most of the veins that drain the pelvis and lower limbs have the same names as the arteries they accompany. All of these veins empty into the inferior vena cava, which returns the blood to the heart.
Blood Pressure and Pulse Measurements
Pulse rate and blood pressure are good indicators of the functioning of the circulatory system. After every systole of the left ventricle, arteries expand and recoil. This creates a moving wave of pressure called the pulse. The pulse rate matches the heart rate, which is from 70 to 80 beats per minute when the body is at rest. Your pulse can be checked in any artery that is close to the skin surface and that can be compressed against a solid structure such as a bone. The pulse rate is commonly checked at the site of the radial artery at the inner wrist or at the site of the carotid artery at the neck. The pulse can also be strongly felt at the femoral artery in the groin, at the brachial artery between the bicep and tricep, and at the dorsalis pedis artery on the top of the foot. The strongest pulse is in arteries near the heart. A rapid resting heart rate, indicated by a rapid pulse rate, of over 100 beats per minute is called tachycardia. A slow resting heart/pulse rate of less than 50 beats per minutes is called bradycardia. Bradycardia is normal in endurance-trained athletes such as marathon runners.
Blood pressure is typically assessed in the brachial artery of the left arm. An inflatable rubber cuff with attached squeezable bulb used to measure BP is called a sphygmomanometer. The bulb is squeezed until compression stops the flow of blood in the brachial artery. Then the cuff is slowly deflated. When the artery reopens, blood spurts through the vein, making a sound that corresponds to systolic blood pressure. With continuing deflation, the sound of the flowing blood ultimately goes away, which corresponds with diastolic blood pressure.
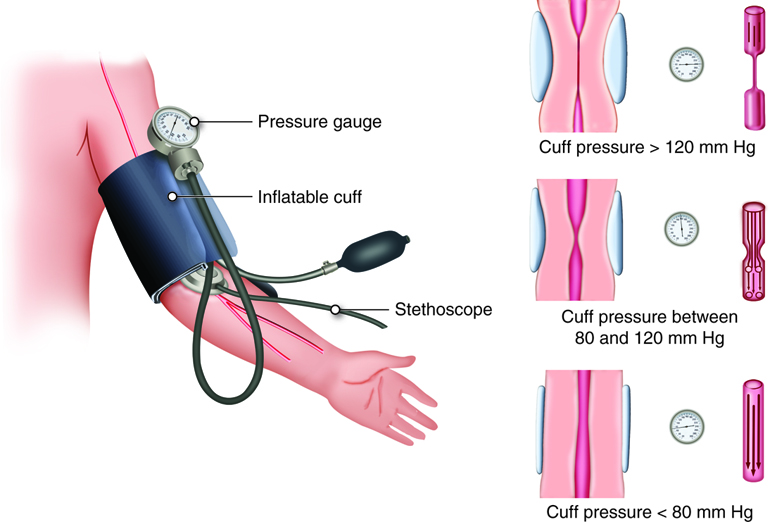
The total volume of circulating blood also affects BP. You may recall the the total amount of blood in the vascular system contributes to venous return. If there is significant bleeding, volume decreases. If the blood volume reduction is less than 10 percent, homeostatic mechanisms kick in to help normalize BP. If blood volume drops by more than 10 percent, the decrease in venous return causes BP to fall. Similarly, conditions that increase blood volume (for example, water retention) usually result in an increased BP – as long as the individual has a healthy heart to pump this extra blood.
Factors Influencing Blood Pressure
Vascular Resistance
Resistance refers to the factors that oppose the flow of blood. Resistance is caused by friction as blood moves through a vessel. The resistance to blood flow as it moves from the aorta to the vena cavae is known as total peripheral (i.e., systemic) resistance. This is in contrast to the pulmonary vascular resistance that the right ventricle pumps into. Although resistance is commonly calculated across the entire circulation, it can also be calculated for a particular type of vessel in the body (such as the systemic arterioles), an organ, or a single specific blood vessel, as long as the pressure drop across the region and the blood flow rate are known.
Vessel Lumen Size
The smaller the lumen, the greater the resistance. Most of the changes in lumen size are due to the vasoconstriction and vasodilation of arteries and arterioles. As a vessel constricts, the lumen size decreases. This increases resistance, which is inversely proportional to the change in radius. This means that even small changes in radius can have large affects on resistance. And, if the flow rate is kept constant when radius decreases, then the pressure change (drop) along the length of the vessel will increase. Because our systemic vasculature is made up of millions of vessels, they each contribute in some way to the overall resistance of the system, called systemic vascular resistance (SVR) or total peripheral resistance.
Blood Viscosity
Viscosity is a measurement of a fluid’s internal resistance (how easily the fluid particles can slide past each other), which determines its ability to flow. Viscosity depends on the thickness or stickiness of a fluid. A fluid with a low viscosity flows quickly; a fluid with a high viscosity flows slowly. When the viscosity is high, it is hard to maintain fluid movement, and molecules cannot freely slip past each other. The viscosity of blood is mostly dependent on the ratio of red blood cells to blood plasma. The more red blood cells in a given volume of blood, the more viscous the blood. The amount of plasma proteins in the blood also affects viscosity. If blood viscosity increases, resistance increases proportionally. If blood flow stays the same, an increased viscosity requires that the heart generate more pressure to move the flow through the system.
Blood Vessel Length
There is a direct correlation between the length of a blood vessel and the amount of resistance. As length increases, so does resistance. This may be obvious if we consider the pulmonary circulation versus the systemic circulation. Each circulation receives the same amount of blood flow, but the pulmonary circulation requires much less pressure to maintain this flow rate. A major reason for this is that the path length for blood flow is much shorter through the pulmonary circulation than it is through the systemic circulation.
Resistance to blood flow increases in people who are overweight because even a kilogram or two of excess fat requires miles of additional small vessels to supply it with blood. This increased vessel length dramatically increases the amount of peripheral resistance. A common consequence of obesity is hypertension.

