34 Muscular Levels of Organization
The force generation required for skeletal muscle function occurs at the molecular level. You can develop a better understanding of the properties of cells and tissues by studying the molecular mechanisms common to the cells involved:
- Molecular level — actin and myosin
- Microscopic level — sarcomere and myofibrils
- Cell level — myoblasts and myofibers
- Tissue level — neuromuscular junctions and fascicles
- Organ level — major skeletal muscles of the body
Molecular Level – Actin and Myosin
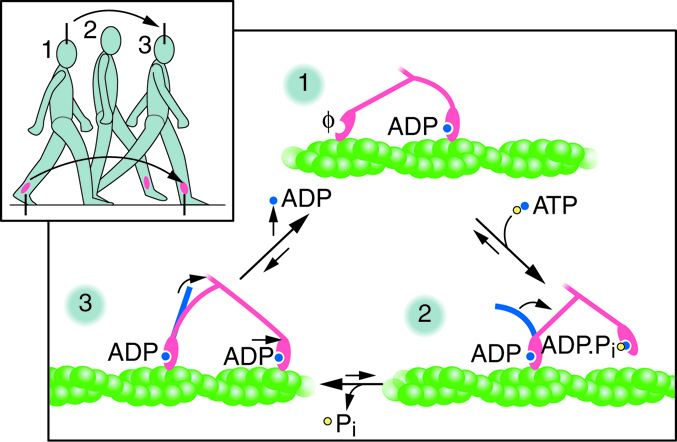
Myosin is a protein that converts the chemical energy stored in the bonds of ATP into the kinetic energy of movement. Myosin is the force-generating protein in all muscle cells, and a coordinated effort among many myosin molecules pulling on actin, generates force for movement. Myosin molecules have two main structural parts. The tail of a myosin molecule consists of two polypeptide subunits wound together, whereas the head is composed of two globular subunits. The tail of a myosin molecule connects with other myosin molecules to form the central region of a thick filament, whereas the heads align on either end of the thick filament where thin filaments overlap with the thick filament. The point at which the head and tail of the molecule meet is flexible and allows the head to move back and forth. This allows myosin to “walk” and pull on actin filaments. Actin filaments are made of individual globular (spherical) protein subunits that assemble linearly into helical (twisted) filaments.
ATP binding to myosin causes it to release actin, allowing actin and myosin to detach from each other. After this occurs, ATP is converted to ADP and Pi. The binding site on myosin that hydrolyzes ATP to ADP is called ATPase. The energy released during ATP hydrolysis changes the angle of the myosin head into a “cocked” position. The myosin head is then in a position for further movement, possessing potential energy; however, ADP and Pi are still attached.
Cross-bridge formation occurs at this point, as actin binds while ADP and Pi are still bound to myosin. Pi is then released, causing myosin to form a stronger attachment to actin, and the myosin head moves toward the M line, pulling actin along with it. As actin is pulled, the filaments move approximately 10 nanometers toward the M line. This movement is called the power stroke, as the thin filament “slides” over the myosin and ADP is released during this step.
When the myosin head is “cocked,” myosin is in a high-energy configuration. This energy is expended as the myosin head moves through the power stroke. At the end of the power stroke, the myosin head is in a low-energy position. Each myosin molecule may form many cross-bridges during muscle contraction. The collection of power strokes and cross-bridges allows collections of individual molecules to generate large forces.
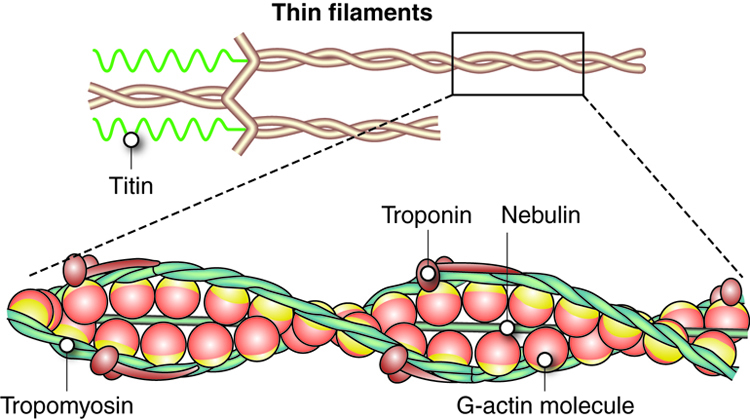
In skeletal muscle, tropomyosin and troponin regulate contraction by controlling the interaction between actin and myosin filaments. Tropomyosin acts to block myosin binding sites on the actin filament, preventing cross-bridge formation and thus preventing contraction in a relaxed muscle. Calcium triggers muscle contraction by binding to troponin and altering its shape so that tropomyosin does not block the myosin binding sites on actin, thus allowing muscle contraction to occur. Calcium is generally an important molecule in muscle function, as we will discuss later.
Titin, as the name implies, is a very large structural protein in muscle cells. Unlike actin and myosin, which bundle together to form a multiprotein complex, titin is a single protein that holds large structures together. Thus, titin is a large, multifunctional protein (hundreds of times bigger than these other proteins) that forms an elastic filament. Titin helps align the myosin proteins and allows the muscle cell to maintain structural integrity by resisting extreme stretching, preventing damage due to overstretching.
Dystrophin is a protein that helps bind actin to the muscle cell membrane. Insufficient dystrophin production results in an inability to transfer the force of the organized actin-myosin contraction to the muscle cell membrane and ultimately to the tendons. Loss or insufficient production of this molecule causes Duchenne muscular dystrophy (DMD).
Example: Rigor and Rigor Mortis
At the end of the power stroke, the actin-myosin cross-bridge is still in place (until ATP binds to the myosin head to change its shape). When energy is depleted, ATP is no longer available to bind to myosin; without ATP, actin remains bound to myosin, making both relaxation and further contraction impossible. This state, with an intact cross-bridge and depleted ATP, is called rigor.
This situation is exemplified during rigor mortis, which occurs after death. Dead cells can no longer produce ATP. Without ATP, the myosin heads can not detach from actin. Recall that ATP is required for the myosin to come off of the actin. Rigor mortis eventually subsides as proteins in the body, including actin and myosin, degrade.
Microscopic Level – The Sarcomere
The fundamental functional unit of muscle is called a sarcomere. One muscle may contain as many as 100,000 of the repeating sarcomere units. In the sarcomere, the myofilaments (thick filaments and thin filaments) are organized into parallel units. Sarcomeres were first identified by imaging (histology), and the nomenclature described below reflects their microscopic “appearance.”
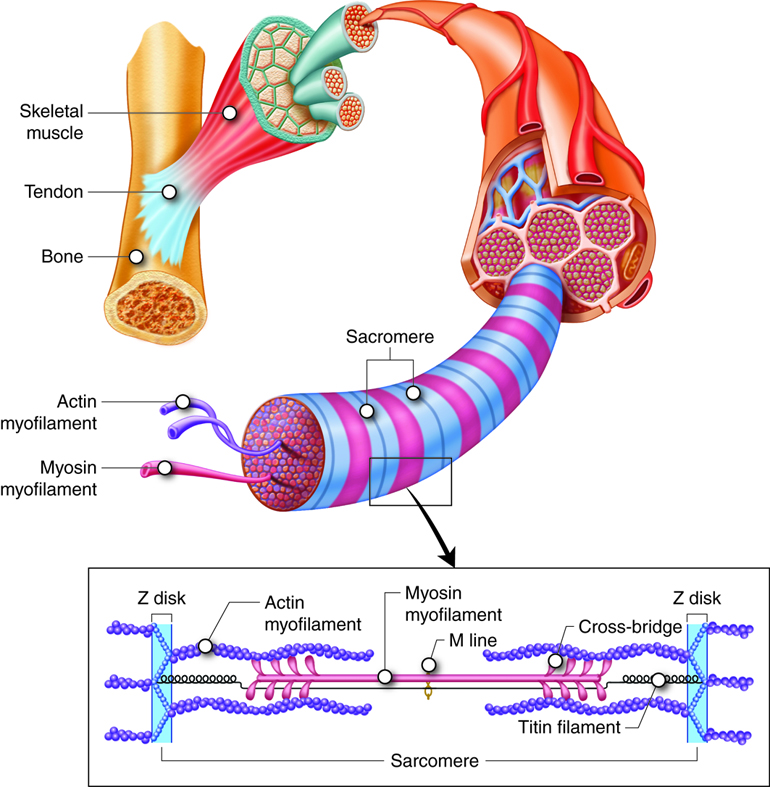
Molecular Organization of Muscle
Myofilaments are organized structures in muscle cells that contain the actin and myosin. The organized globular proteins of actin in muscle cells form a thin filament, and bundles of over 200 myosin proteins form a thick filament. The thick filament myosin heads “walk” along the actin thin filaments. A single thin filament is composed of 300-400 globular actins with 40-60 troponin and tropomyosin molecules. A single thick filament is composed of more than 200 myosin molecules.
Actin filaments are thin, causing the actin “rope” to appear skinny. The myosin filament contains many myosins bundled up with all of the head groups sticking out, so that it looks “fluffy.” That fluffiness makes it look thick.
Sarcomere Anatomy—H Zone, M Line, Z Disc, I Band and A Band
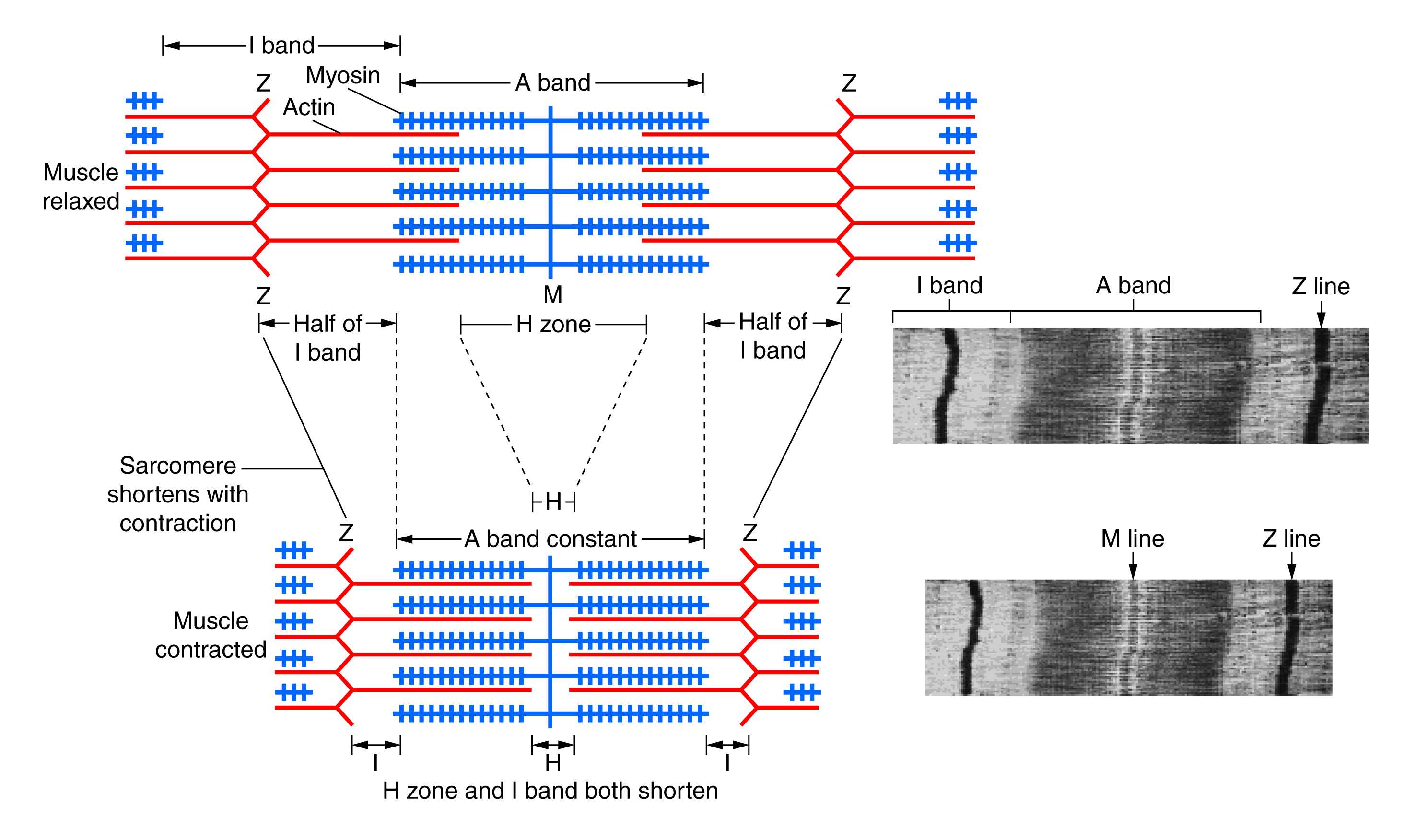
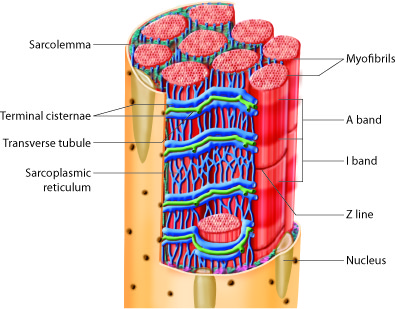
Histological sections of muscle show the anatomical features of the sarcomeres. Thick filaments, composed of myosin, are visible as the A band of a sarcomere. Thin filaments, composed of actin, attach to a protein in the Z disc (or Z line) called alpha-actinin, and they are present across the entire length of the I band and a portion of the A band. The region where thick and thin filaments overlap has a dense appearance, as there is little space between the filaments. This zone where thin and thick filaments overlap is very important to muscle contraction, as it is the site where filament movement starts. Thin filaments do not extend completely into the A bands, leaving a central region of the A band that only contains thick filaments. This central region of the A band looks slightly lighter than the rest of the A band, and is called the H zone. The middle of the H zone has a vertical line called the M line, where accessory proteins hold together thick filaments.
| Microscopically Visible Feature | Composition |
|---|---|
| A band | Region of thick-filament myosin proteins |
| H zone | Central region of the A band with no overlapping actin proteins when muscle is relaxed |
| I band | Region of thin-filament actin proteins, with no myosin |
| M line | M line accessory proteins in center of myosin thick filament perpendicular to the sarcomere |
| Z disc | Zig-zag line of Z line proteins and actin binding proteins perpendicular to the sarcomere |
Microscopic Level – Organelles and Cell Structures
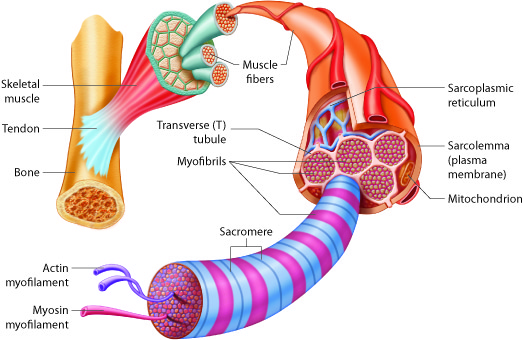
Muscle cells contain organelles found in all cells, including nuclei, the endoplasmic reticulum, mitochondria and the Golgi apparatus. The amount and organization of organelles and structures is slightly different in muscle cells. Actin is found inside every cell in the body, but actin is specially organized within the sarcomeres of muscle cells. Muscle cells also have extremely high numbers of mitochondria to produce ATP for force generation. Recall that an ATP molecule is required for one myosin to perform one power stroke.
Organelles and Structures Specific to Muscle Cells
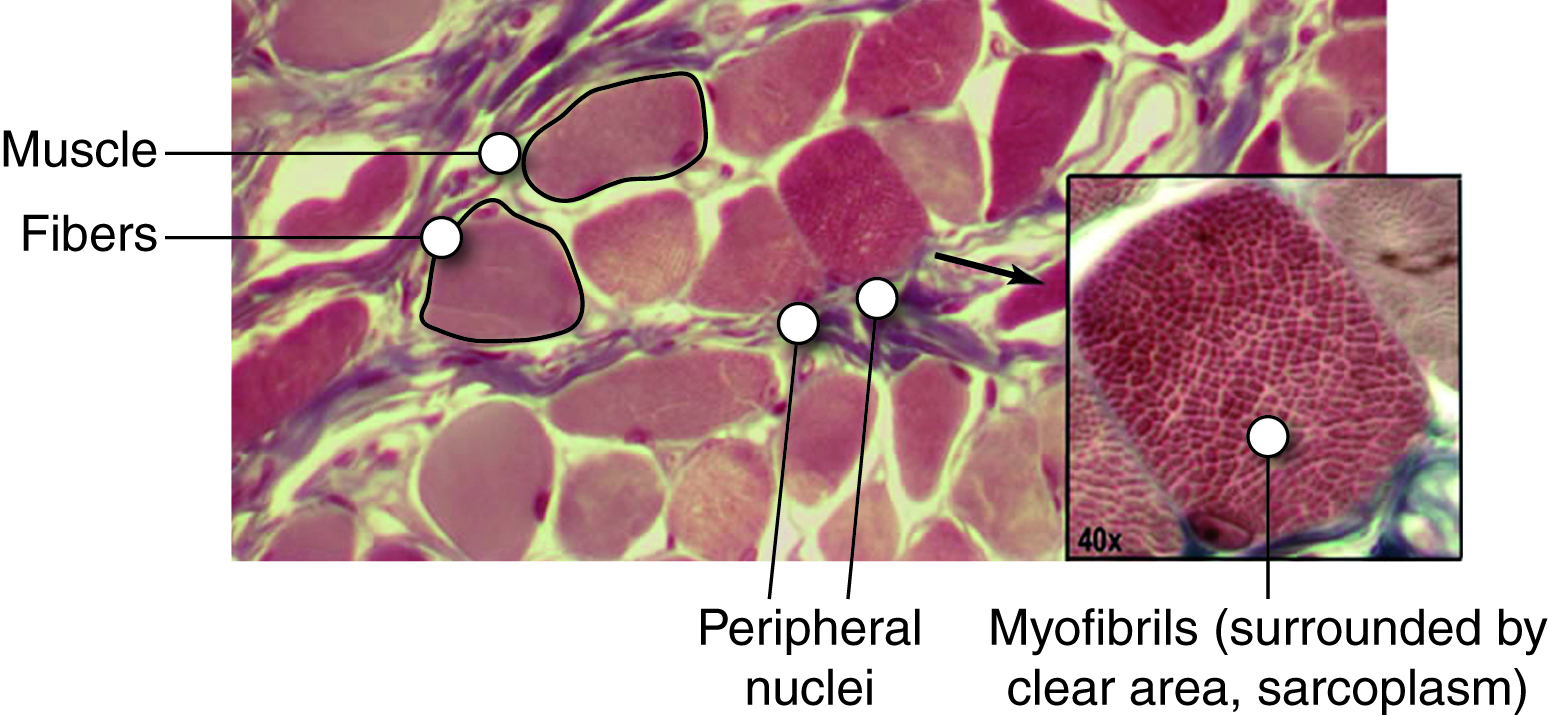
The image is a light microscopic image of skeletal muscle in cross section showing a group muscle cells, each surrounded by epimysium and the group surrounded by perimysium. Blood vessels are seen passing through the image and in cross section on the right. The magnified image shows cross section of a single cell (fiber) at higher magnification with many myofibrils visible (small dark circles).
Each skeletal muscle fiber is a single cell produced from the fusion of many precursor cells. These fused cells are therefore functionally quite large, with diameters of up to 100 micrometers and lengths of up to 30 centimeters. Compare this with a cell of the skin which is a cube of 20 micrometers in nearly every diameter. In muscle fibers, sarcomeres arrange into parallel structures called myofibrils, so both the Z disc and the M line hold myofilaments in place to maintain the structural arrangement and layering. Myofibrils are connected to each other by intermediate, or desmin, filaments that attach to the Z disc.
There is some special nomenclature associated with these fused cells. Each skeletal muscle fiber cell has more than one nucleus, which is called multinucleate. The plasma membrane of this fused skeletal muscle cell is called the sarcolemma. The muscle interacts with the nerves that stimulate the muscle at the sarcolemma, and we will describe this interaction later. Within the sarcolemma is the sarcoplasm, which is the cytoplasm of the fused muscle fiber. The sarcoplasm has most of the same components as standard cytoplasm in addition to high levels of the protein myoglobin, which stores oxygen.
Also, the sarcolemma contains many structures similar to the plasma membrane of other cells, but it also possesses structures unique to muscle cells. The sarcolemma has transverse tubules, or T tubules, which are indentations of the sarcolemma into the interior of the cell along the length of the muscle cell. The T tubules are filled with extracellular fluid, and they conduct the action potential from the nerve deep into the interior of the muscle cells, which can be very large. With T-tubules, nerves can stimulate entire muscles and muscle groups quickly and effectively. Without T tubules, action potential conduction into the interior of the cell would happen much more slowly, causing delays between neural stimulation and muscle contraction, resulting in slower, weaker contractions.
Inside skeletal muscle fibers is a network of membranous tubules called the sarcoplasmic reticulum (SR), which is similar to the smooth endoplasmic reticulum found in other cells. SR tubules are filled with high-calcium fluid, and they surround each myofibril. Terminal cisternae are dilated regions of the SR that form on either side of each T tubule extending into the cell. A grouping consisting of a T tubule, from the outside of the muscle fiber, and two terminal cisternae, from the inside of the muscle fiber, is called a triad.
T tubules conduct an action potential along the surface of the muscle fiber into triads that trigger the release of Ca2+ ions from the nearby terminal cisternae. This, in turn, triggers muscle contraction when the calcium ions in the sarcoplasm can bind to the troponin of the sarcomeres.
Skeletal Muscle Cells
Skeletal Myocytes—Myoblast Fusion, Myotube Organization in Skeletal Muscle Tissue and Satellite Cells
Each skeletal muscle fiber is a single skeletal muscle cell, also known as a skeletal myocyte (“myo-” refers to “muscle” and “-cyte” refers to “cell”), that is formed from the fusion of precursor cells. As described before, cell fusion leads to multinucleation of each mature muscle fiber. Each myoblast, the embryonic cell type that differentiates into muscle, contributes one nucleus when the muscle fiber is formed during development.
During development, individual myoblasts (“-blast” refers to “building” … like osteoblasts), migrate to different regions in the body and then fuse to form a myotube. A myotube is a type of syncytium, which is the term used for a group of fused cells. Skeletal muscle cells are multinucleate because the syncytium (“syn-” means “same” and “cyt” refers to “cytoplasm”) fusion retains the nucleus of each contributing myoblast. This syncytium leads to the collective sarcoplasm and sarcolemma, described above.
Mature muscle does not grow by this process. Mature cells can change in size, but new cells are not formed when muscles grow. Instead, structural proteins are added to muscle fibers in a process called hypertrophy. The reverse, when structural proteins are lost and muscle mass decreases, is called atrophy. Cellular components of muscles can also undergo changes in response to changes in muscle use.
Although the number of muscle cells is set during development, satellite cells help to repair skeletal muscle fibers. Satellite cells are similar to myoblasts in that they are able to divide, fuse and differentiate. These satellite cells are located outside the muscle fibers and are stimulated to grow and fuse with muscle cells by growth factors that are released by muscle fibers under certain forms of stress. Satellite cells can regenerate muscle fibers to a very limited extent, but they primarily help to repair damage in living cells. Satellite cells facilitate the protein synthesis required for repair and growth. If a cell is damaged to a greater extent than can be repaired by satellite cells, the muscle fibers are replaced by scar tissue in a process called fibrosis. Because scar tissue cannot contract, muscle that has sustained significant damage loses strength and cannot produce the same amount of force or endurance as it could before being damaged.
Cardiac Muscle Cells (Myocytes)
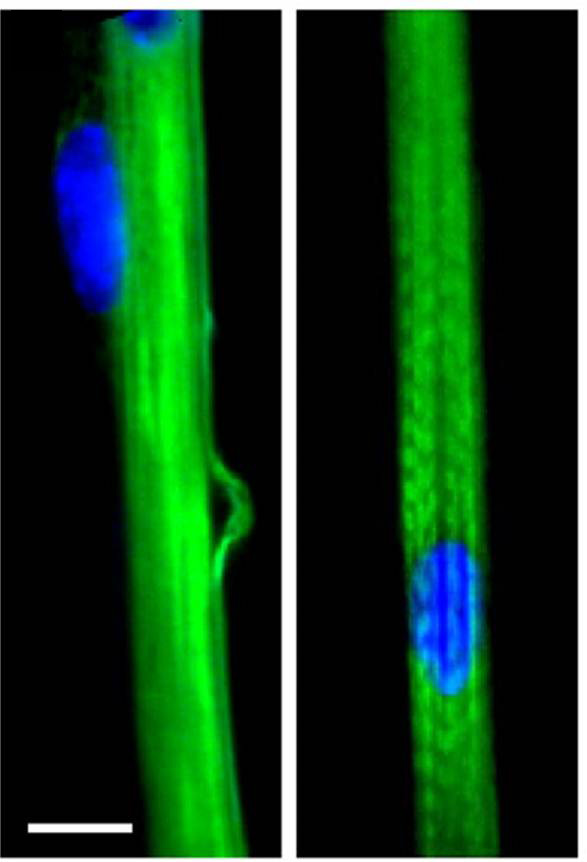
Cardiac tissue is only found in the walls of the heart chambers, where it provides the muscle contractions required to pump blood throughout the body. At the tissue level, cardiac muscle is striated (or striped) since, like skeletal muscle, it has organized sarcomeres. However, cardiac muscle fibers are shorter than skeletal muscle fibers and usually contain only one nucleus, which is located in the central region of the cell.
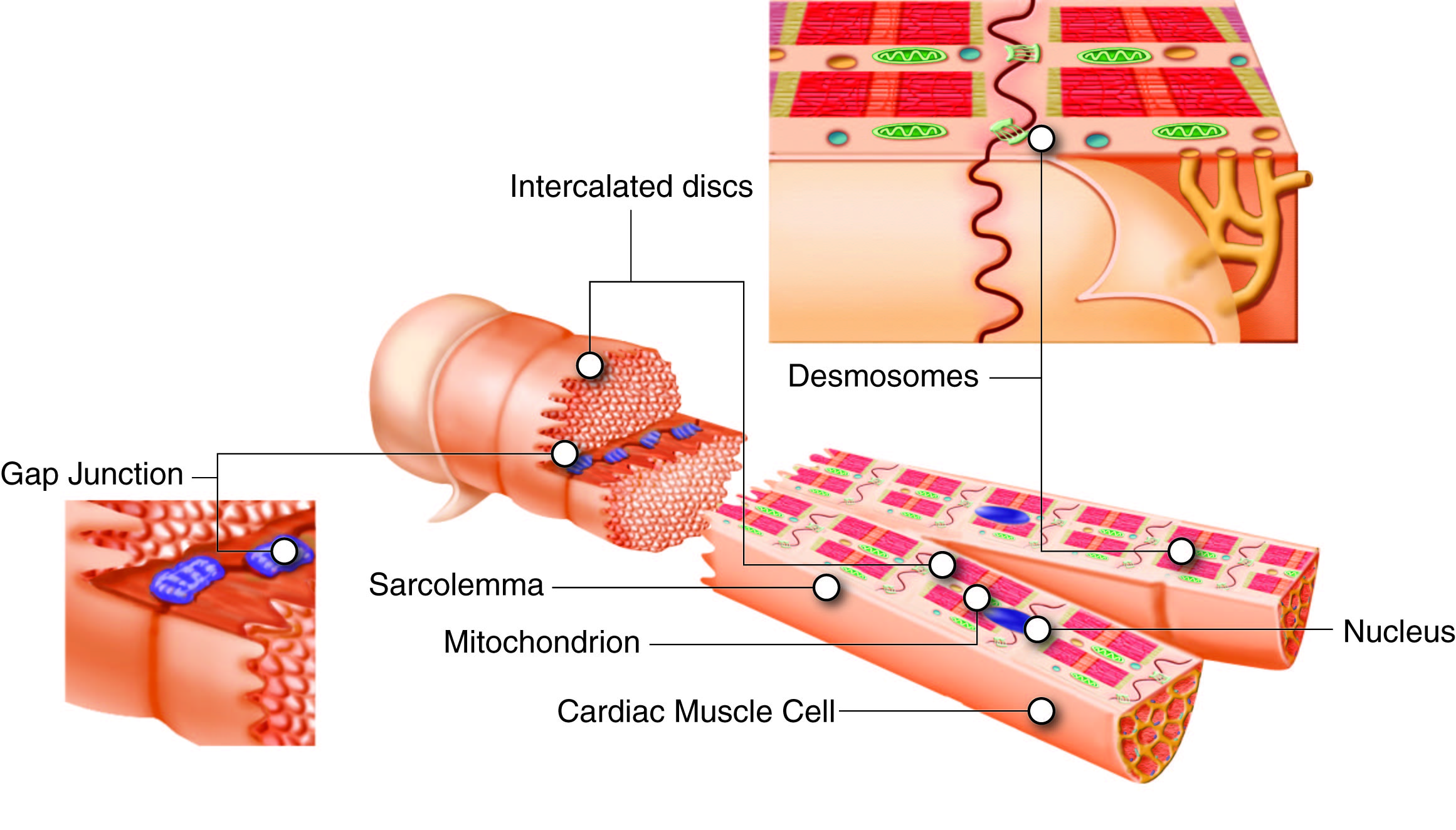
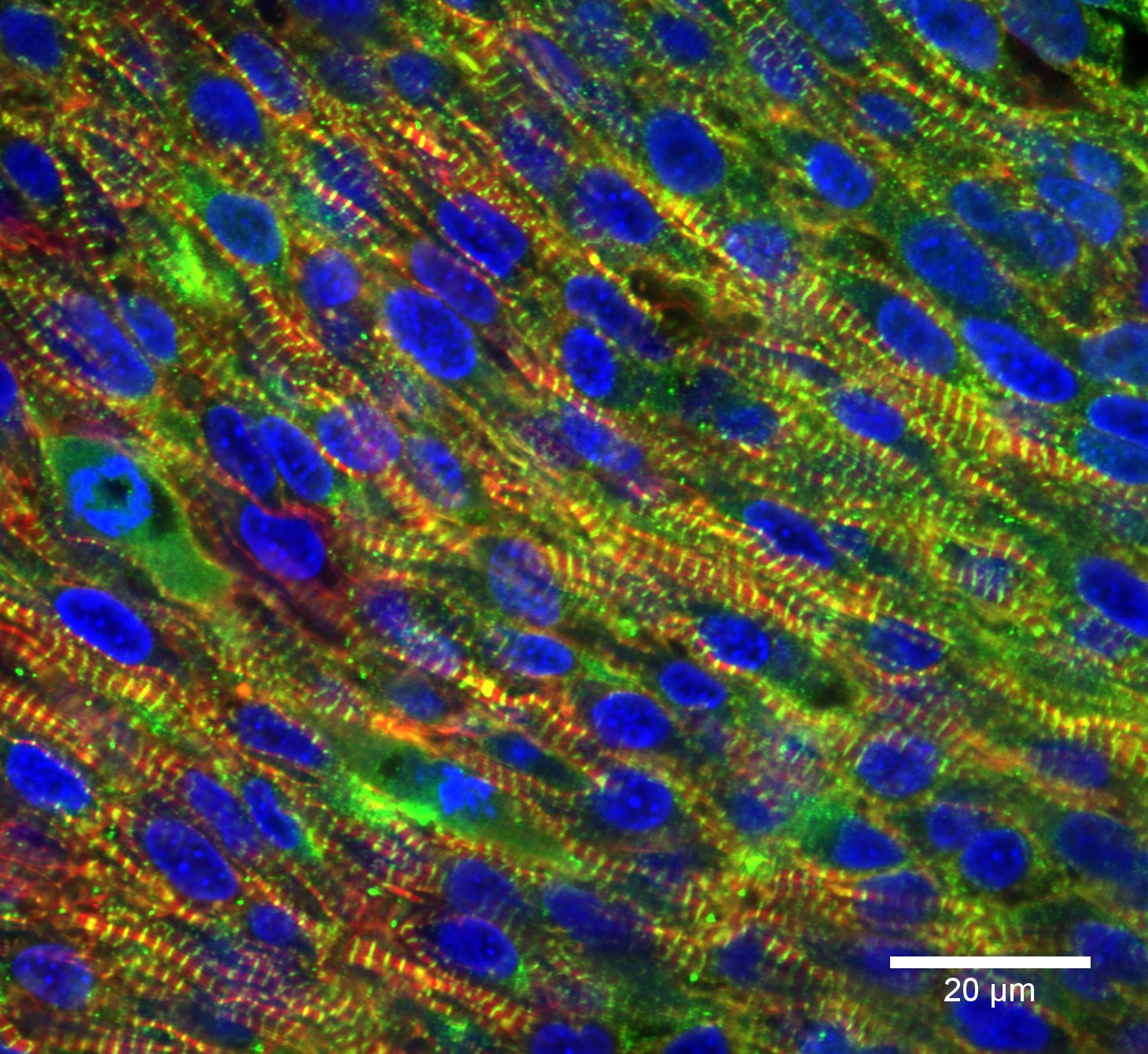
Cardiac muscle fibers are branched, whereas skeletal muscle fibers are unbranched. This branching allows individual cells to contact several adjacent cells at specialized cell junctions called intercalated discs. Intercalated discs are part of the sarcolemma and contain two structures important in cardiac muscle contraction: gap junctions and desmosomes. Gap junctions are tunnels or protein channels in the cell membrane connecting adjacent cardiac muscle cells, allowing ions involved in electric currents to move quickly from one cell to the next. This is called electric coupling, and in cardiac muscles, it allows the quick transmission of action potentials and synchronized contraction of the entire heart. Desmosomes are especially strong cell-to-cell junctions that help maintain structural integrity at the connections between these contractile cardiac muscle cells.
Cardiac Cell Specialization
There are two specialized types of cardiac cells: the contractile cells and the pacemaker cells. The contractile cells that produce the force for the beating of the heart have the capability to beat on their own, but for useful organ level contractions, the cells must beat as a unit. The stimulus for contraction is normally provided by the pacemaker cells and this stimulus is passed through gap junctions to synchronize the signals. These electrically conductive pacemaker cells are important for electrical stimulation since cardiac muscle cells are not under voluntary control. Pacemaker cells are spontaneously depolarizing at set intervals (faster than contractile cells would do on their own), starting a wave of depolarization that then spreads throughout the heart and triggers contraction. Because the pacemaker cells are located in the heart, the heart is said to control its own contraction, which is called autorhythmicity (or automaticity). Pacemaker cells depolarize at set intervals, and the heart beats a steady, predictable 60 to 80 beats per minute at rest. These repetitive contractions ensure a constant blood supply to all body cells.
Smooth Muscle Cells
Smooth muscle tissue is found in many different body systems, including as part of organs in the digestive, respiratory, and reproductive tracts and in the walls of blood vessels. Smooth muscle cells are approximately the same size as cardiac muscle cells and also have only one nucleus. However, smooth muscle cells are not branched and, unlike both cardiac and skeletal muscle, smooth muscle cells don’t have sarcomeres. Smooth muscle cells form layers that are usually arranged so that one runs parallel to an organ and the other wraps around it. These two muscle layers then contract in turn, causing alternating dilation and contraction or lengthening and shortening of the organ, moving substances through internal passages. This is called peristalsis and is displayed in the process of digestion as food moves through the gastrointestinal tract.
Similar to skeletal and cardiac muscle cells, smooth muscle can undergo hypertrophy to increase in size. Unlike the other two muscle types, mature smooth muscle cells can also divide to produce more cells, a process called hyperplasia. This can be observed in the uterus, which responds to increased estrogen levels by producing more uterine muscle cells.
Smooth muscle cells also do not possess T tubules and do not have a very extensive sarcoplasmic reticulum. Smooth muscle has actin and myosin but they are not organized into sarcomeres, so there are no obvious bands or striations. Instead, actin and myosin is organized into dense bodies attached to the sarcolemma, shortening the muscle cell as thin filaments slide past thick filaments. Thin and thick filaments are aligned in a diagonal pattern across the cell so that contraction produces a twisting or corkscrew motion, rotating one way as it contracts and the other way as it relaxes. Cross-bridge formation and filament sliding processes are the same in smooth muscle as they are in skeletal and cardiac muscle. Actin, myosin, and tropomyosin are all present, but smooth muscle cells do not possess troponin as their regulatory protein. Instead, a molecule called calmodulin binds to calcium and activates myosin cross-bridge formation. There is also a greater ratio of actin to myosin in smooth muscle, meaning that there are more thin filaments for every thick filament.
Most smooth muscles must function for long periods without rest, so their power output is relatively low, but contractions can continue without utilizing large amounts of energy. This occurs because the ATPase in myosin works at a relatively slow rate, meaning that high levels of ATP are not available for powerful contractions but a steady supply is produced for sustained contractions. Smooth muscle can also maintain contractions through a latch state, during which actin and myosin remain locked together, or latched, in the absence of Ca2+ ions. This does not require ATP, thereby producing sustained contractions without using energy. This allows smooth muscles to keep your blood vessels partially contracted for your entire life without them fatiguing.
Pacesetter cells
Similar to cardiac muscle, smooth muscle is not under voluntary control. In addition to spontaneous stimulation, smooth muscle can be stimulated by pacesetter cells that are similar to pacemaker cells and trigger waves of action potentials in smooth muscle. Smooth muscle can also be stimulated by the autonomic nervous system or hormones. Neuromuscular junctions are not present in smooth muscle, but varicosities, enlargements along autonomic nerves, release neurotransmitters into synaptic clefts. Smooth muscle can respond to a variety of neurotransmitters to produce different effects at different locations.
Smooth muscle can be divided into two types based on how depolarization and muscle contraction occur. Single-unit smooth muscle cells contain gap junctions, which allow the cells to be electrically coupled. Electric couplings allow action potentials to spread quickly from one cell to the next, permitting coordinated depolarization and contraction. In this manner, groups of muscle cells act as a single unit, contracting in unison. This type of smooth muscle is found in hollow organs, including the gastrointestinal tract, and in the walls of small blood vessels, and it is often stimulated spontaneously or by stretching, to produce an action potential.
Multiunit smooth muscle cells rarely possess gap junctions, so they are not electrically coupled. Stimuli for multiunit smooth muscles come from autonomic nerves or hormones but not from stretching. This type of tissue is found in the walls of large blood vessels, in the respiratory airways, and connected to hair follicles (to make your hair “stand up”), among other places.
| Skeletal Muscle | Cardiac Muscle | Smooth Muscle | |
|---|---|---|---|
| Location in the body | Primarily attached to bones | Only in the heart | Walls of blood vessels and visceral organs |
| Cellular anatomy | Large fibers of multinucleated cells with sarcomere striations | Mononucleated branched cells with sarcomere striations | Single mononucleated cells with no striations |
| Other anatomical features | Connective tissues within, between and around fibers | Connection to fibers of the heart | Loose connections within a fascicle |
Neuromuscular Junctions
Skeletal muscle cell contraction occurs after a release of calcium ions from internal stores, which is initiated by a neural signal. Each skeletal muscle fiber is controlled by a motor neuron, which conducts signals from the brain or spinal cord to the muscle.
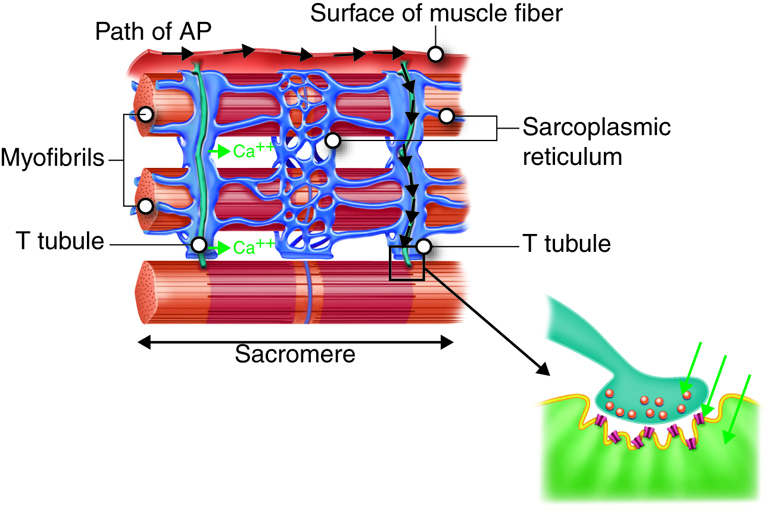
The following list presents an overview of the sequence of events involved in the contraction cycle of skeletal muscle:
- The action potential travels down the neuron to the presynaptic axon terminal.
- Voltage-dependent calcium channels open and Ca2+ ions flow from the extracellular fluid into the presynaptic neuron’s cytosol.
- The influx of Ca2+ causes neurotransmitter (acetylcholine)-containing vesicles to dock and fuse to the presynaptic neuron’s cell membrane.
- Vesicle membrane fusion with the nerve cell membrane results in the emptying of the neurotransmitter into the synaptic cleft; this process is called exocytosis.
- Acetylcholine diffuses into the synaptic cleft and binds to the nicotinic acetylcholine receptors in the motor end-plate.
- The nicotinic acetylcholine receptors are ligand-gated cation channels, and open when bound to acetylcholine.
- The receptors open, allowing sodium ions to flow into the muscle’s cytosol.
- The electrochemical gradient across the muscle plasma membrane causes a local depolarization of the motor end-plate.
- The receptors open, allowing sodium ions to flow into and potassium ions to flow out of the muscle’s cytosol.
- The electrochemical gradient across the muscle plasma membrane (more sodium moves in than potassium out) causes a local depolarization of the motor end-plate.
- This depolarization initiates an action potential on the muscle fiber cell membrane (sarcolemma) that travels across the surface of the muscle fiber.
- The action potentials travel from the surface of the muscle cell along the membrane of T tubules that penetrate into the cytosol of the cell.
- Action potentials along the T tubules cause voltage-dependent calcium release channels in the sarcoplasmic reticulum to open, and release Ca2+ ions from their storage place in the cisternae.
- Ca2+ ions diffuse through the cytoplasm where they bind to troponin, ultimately allowing myosin to interact with actin in the sarcomere; this sequence of events is called excitation-contraction coupling.
- As long as ATP and some other nutrients are available, the mechanical events of contraction occur.
- Meanwhile, back at the neuromuscular junction, acetylcholine has moved off of the acetylcholine receptor and is degraded by the enzyme acetylcholinesterase (into choline and acetate groups), causing termination of the signal.
- The choline is recycled back into the presynaptic terminal, where it is used to synthesize new acetylcholine molecules.
This video also explains how muscle contraction is signaled:
Dongem Biology. (2009). How a muscle contraction is signaled – Animation. https://www.youtube.com/watch?v=CepeYFvqmk4&feature=emb_logo
The Anatomy and Physiology of the Neuromuscular Junction
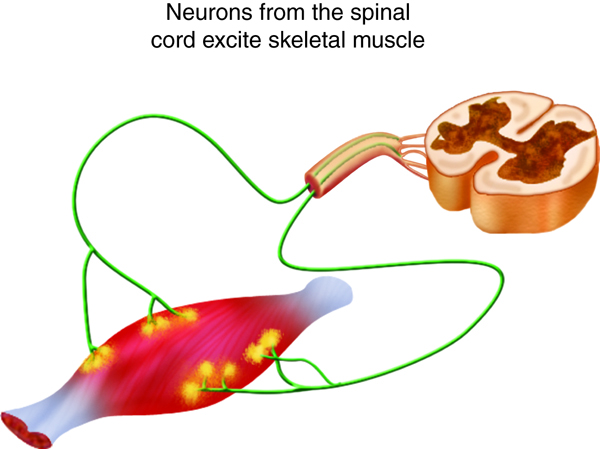
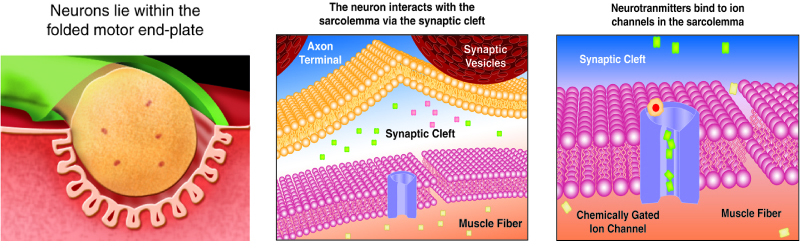
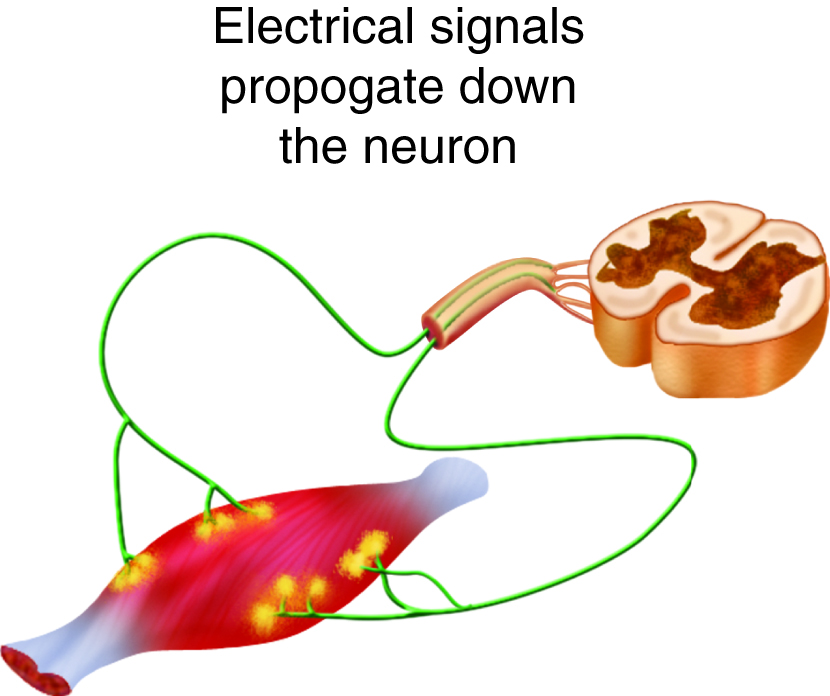
We stimulate skeletal muscle contraction voluntarily. Electrical signals from the brain through the spinal cord travel through the axon of the motor neuron. The axon then branches through the muscle and connects to the individual muscle fibers at the neuromuscular junction. The folded sarcolemma of the muscle fiber that interacts with the neuron is called the motor end-plate; the folded sarcolemma increases surface area contact with receptors. The ends of the branches of the axon are called the synaptic terminals, and do not actually contact the motor end-plate. A synaptic cleft separates the synaptic terminal from the motor end-plate, but only by a few nanometers.
Communication occurs between a neuron and a muscle fiber through neurotransmitters. Neural excitation causes the release of neurotransmitters from the synaptic terminal into the synaptic cleft, where they can then bind to the appropriate receptors on the motor end-plate. The motor end-plate has folds in the sarcolemma, called junctional folds, that create a large surface area for the neurotransmitter to bind to receptors. Generally, there are many folds and invaginations that increase surface area including junctional folds at the motor endplate and the T-tubules throughout the cells.
Physiology
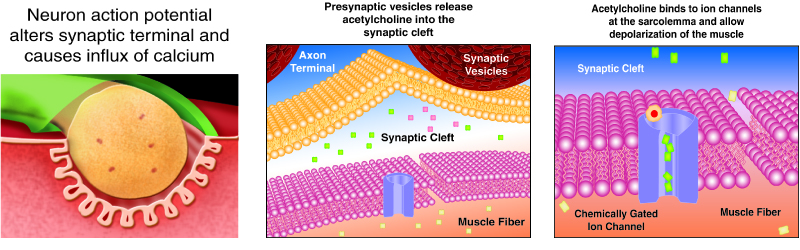

The neurotransmitter acetylcholine is released when an action potential travels down the axon of the motor neuron, resulting in altered permeability of the synaptic terminal and an influx of calcium into the neuron. The calcium influx triggers synaptic vesicles, which package neurotransmitters, to bind to the presynaptic membrane and to release acetylcholine into the synaptic cleft by exocytosis.
The balance of ions inside and outside a resting membrane creates an electric potential difference across the membrane. This means that the inside of the sarcolemma has an overall negative charge relative to the outside of the membrane, which has an overall positive charge, causing the membrane to be polarized. Once released from the synaptic terminal, acetylcholine diffuses across the synaptic cleft to the motor end-plate, where it binds to acetylcholine receptors, primarily the nicotinic acetylcholine receptors. This binding causes activation of ion channels in the motor end-plate, which increases permeability of ions via activation of ion channels: sodium ions flow into the muscle and potassium ions flow out. Both sodium and potassium ions contribute to the voltage difference while ion channels control their movement into and out of the cell. As a neurotransmitter binds, these ion channels open, and Na+ ions enter the membrane. This reduces the voltage difference between the inside and outside of the cell, which is called depolarization. As acetylcholine binds at the motor-end plate, this depolarization is called an end-plate potential. It then spreads along the sarcolemma, creating an action potential as voltage-dependent (voltage-gated) sodium channels adjacent to the initial depolarization site open. The action potential moves across the entire cell membrane, creating a wave of depolarization.
After depolarization, the membrane needs to be returned to its resting state. This is called repolarization, during which sodium channels close and potassium channels open. Because positive potassium ions (K+) move from the intracellular space to the extracellular space, this allows the inside of the cell to again become negatively charged relative to the outside. During repolarization, and for some time after, the cell enters a refractory period, during which the membrane cannot become depolarized again. This is because in order to have another action potential, sodium channels need to return to their resting state, which requires an intermediate step with a delay.
Propagation of an action potential and depolarization of the sarcolemma comprise the excitation portion of excitation-contraction coupling, the connection of electrical activity and mechanical contraction. The structures responsible for coupling this excitation to contraction are the T tubules and sarcoplasmic reticulum (SR). The T tubules are extensions of the sarcolemma and thus carry the action potential along their surface, conducting the wave of depolarization into the interior of the cell. T tubules form triads with the ends of two SR called terminal cisternae. SRs, and especially terminal cisternae, contain high concentrations of Ca2+ ions inside. As an action potential travels along the T tubule, the nearby terminal cisternae open their voltage-dependent calcium release channels, allowing Ca2+ to diffuse into the sarcoplasm. The influx of Ca2+ increases the amount of calcium available to bind to troponin. Troponin bound to Ca2+ undergoes a conformational change that results in tropomyosin moving on the actin filament. When tropomyosin moves, the myosin binding site on the actin is uncovered. This continues as long as excess Ca2+ is available in the sarcoplasm. When there is no more free Ca2+ available to bind to troponin, the contraction will stop. To restore Ca2+levels back to a resting state, the excess Ca2+ is actively transported back into the SR. In a resting state, Ca2+ is retained inside the SR, keeping sarcoplasmic Ca2+ levels low. Low sarcoplasmic calcium levels prevent unwanted muscle contraction.
Neurotransmitters
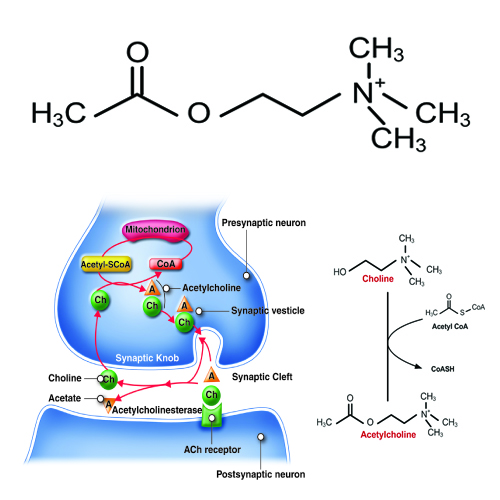
Acetylcholine, often abbreviated as ACh, is a neurotransmitter released by motor neurons that binds to receptors in the motor end-plate. It is an extremely important small molecule in human physiology. On the neuron side of the synaptic cleft, there are typically 300,000 vesicles waiting to be exocytosed at any time and each vesicle contains up to 10,000 molecules of acetylcholine.
ACh is produced by the reaction of Acetyl coenzyme A (CoA) with a choline molecule in the neuron cell body. After it is packaged, transported, and released, it binds to the acetylcholine receptor on the motor end-plate; it is degraded in the synaptic cleft by the enzyme acetylcholinesterase (AChE) into acetate (and acetic acid) and choline. The choline is recycled back into the neuron. AChE resides in the synaptic cleft, breaking down ACh so that it does not remain bound to ACh receptors, which would interrupt normal control of muscle contraction. In some cases, insufficient amounts of ACh prevent normal muscle contraction and cause muscle weakness.
Example: Botulinum’s Effect on ACh
Botulinum toxin prevents ACh from being released into the synaptic cleft. With no ACh binding to its receptors at the motor end-plate, no action potential is produced, and muscle contraction cannot occur. Botulinum toxin is produced by Clostridium botulinum, a bacterium sometimes found in improperly canned foods. Ingestion of very small amounts can cause botulism, which can cause death due to the paralysis of skeletal muscles, including those required for breathing.
Cellular Muscle Contraction
ATP supplies the energy for muscle contraction to take place. In addition to its direct role in the cross-bridge cycle, ATP also provides the energy for the active-transport Na+/K+ and Ca2+ pumps. Muscle contraction does not occur without sufficient amounts of ATP. The amount of ATP stored in muscle is very low, only sufficient to power a few seconds worth of contractions. As it is broken down, ATP must therefore be regenerated and replaced quickly to allow for sustained contraction. One ATP moves one myosin head one step.
There are three mechanisms by which ATP can be regenerated: creatine phosphate metabolism, anaerobic glycolysis, and aerobic respiration.
Creatine phosphate is a phosphagen, which is a compound that can store energy in its phosphate bonds. In a resting muscle, excess ATP (adenosine triphosphate) transfers its energy to creatine, producing ADP (adenosine diphosphate) and creatine phosphate. When the muscle starts to contract and needs energy, creatine phosphate and ADP are converted into ATP and creatine by the enzyme creatine kinase. This reaction occurs very quickly; thus, phosphagen-derived ATP powers the first few seconds of muscle contraction. However, creatine phosphate can only provide approximately 15 seconds worth of energy, at which point another energy source has to be available.
After the available ATP from creatine phosphate is depleted, muscles generate ATP using glycolysis. Glycolysis is an anaerobic process that breaks down glucose (sugar) to produce ATP; however, glycolysis cannot generate ATP as quickly as creatine phosphate. The sugar used in glycolysis can be provided by blood glucose or by metabolizing glycogen that is stored in the muscle. Each glucose molecule produces two ATP and two molecules of pyruvate, which can be used in aerobic respiration or converted to lactic acid.
If oxygen is available, pyruvic acid is used in aerobic respiration. However, if oxygen is not available, pyruvic acid is converted into lactic acid, which may contribute to muscle fatigue and pain. This occurs during strenuous exercise when high amounts of energy are needed but oxygen cannot be delivered to muscle at a rate fast enough to meet the whole need. Anaerobic glycolysis cannot be sustained for very long (approximately one minute of muscle activity), but it is useful in facilitating short bursts of high-intensity output. Glycolysis does not utilize glucose very efficiently, producing only two ATP molecules per molecule of glucose, and the by-product lactic acid contributes to muscle fatigue as it accumulates.
Aerobic respiration is the breakdown of glucose in the presence of oxygen to produce carbon dioxide, water, and ATP. Aerobic respiration in the mitochondria of muscles uses glycogen from muscle stores, blood glucose, pyruvic acid, and fatty acids. Approximately 95 percent of the ATP required for resting or moderately active muscles is provided by aerobic respiration. Aerobic respiration is much more efficient than anaerobic glycolysis, producing approximately 38 ATP molecules per molecule of glucose. However, aerobic respiration does not synthesize ATP as quickly as anaerobic glycolysis, meaning that the power output of muscles declines, but lower-power contractions can be sustained for longer periods.
This combination of different energy sources is important for different types of muscle activity. As an analogy, a cup of coffee with lots of sugar provides a quick burst of energy but not for very long. A balanced meal with complex carbohydrates, protein and fats takes longer to impact us, but provides sustained energy.
Muscles require a large amount of energy, and thus require a constant supply of oxygen and nutrients. Blood vessels enter muscle at its surface, after which they are distributed through the entire muscle. Blood vessels and capillaries are found in the connective tissue that surrounds muscle fascicles and fibers, allowing oxygen and nutrients to be supplied to muscle cells and metabolic waste to be removed. Myoglobin, which binds oxygen similarly to hemoglobin and gives muscle its red color, is found in the sarcoplasm.
Example: Exercise and Energy
After the first few seconds of exercise, available ATP is used up. After the next few minutes, cellular glucose and glycogen are depleted. After the next 30 minutes, the body’s supply of glucose and glycogen are depleted. After that time, fatty acids and other energy sources are used to make ATP. That’s why we should exercise for more than 30 minutes to lose weight (i.e. lose fat). Sometimes, time is important.
Sarcomere Contraction
You have already learned about the anatomy of the sarcomere, with its coordinated actin thin filaments and myosin thick filaments. For a muscle cell to contract, the sarcomere must shorten in response to a nerve impulse. The thick and thin filaments do not shorten, but they slide by one another, causing the sarcomere to shorten while the filaments remain the same length. This process is known as the sliding filament model of muscle contraction. The mechanism of contraction is accomplished by the binding of myosin to actin, resulting in the formation of cross-bridges that generate filament movement.
When a sarcomere shortens, some regions shorten while others remain the same length. A sarcomere is defined as the distance between two consecutive Z discs or Z lines. When a muscle contracts, the distance between the Z discs is reduced. The H zone, the central region of the A zone, contains only thick filaments and shortens during contraction. The I band contains only thin filaments and also shortens. The A band does not shorten; it remains the same length, but A bands of adjacent sarcomeres move closer together during contraction. Thin filaments are pulled by the thick filaments towards the center of the sarcomere until the Z discs approach the thick filaments. The zone of overlap, where thin filaments and thick filaments occupy the same area, increases as the thin filaments move inward.

With large numbers of relatively weak molecular motors, we can more easily adjust the force to meet our needs. Otherwise, we would regularly be producing too little or too much force for most of our tasks. Also, molecules are only capable of generating small forces based on their molecular structure.
Neural Stimulation of Contraction
You have already learned about how the information from a neuron ultimately leads to a muscle cell contraction.
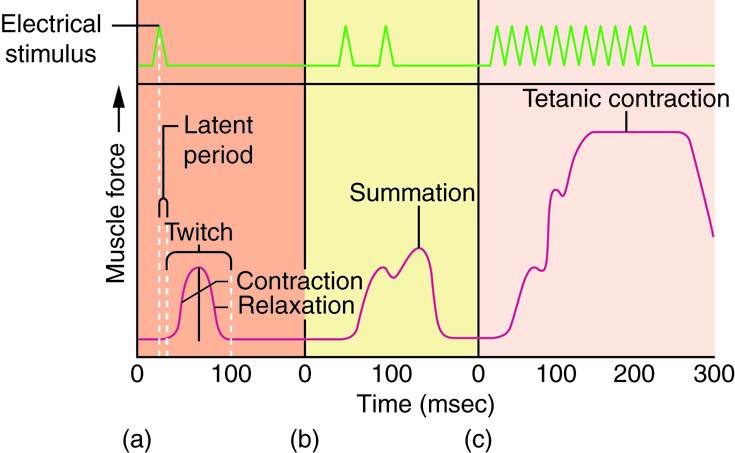
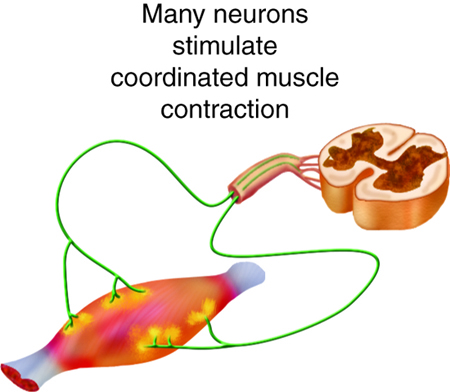
One action potential in a motor neuron produces one contraction. This contraction is called a twitch. We think of “muscle twitches” as spasms that we can’t control, but in physiology, a twitch is a technical term describing a muscle response to stimulation. A single twitch does not produce any significant muscle contraction. Multiple action potentials (repeated stimulation) are needed to produce a muscle contraction that can produce work. Normal muscle contraction is more sustained, and it can be modified to produce varying amounts of force. This is called a graded muscle response. The tension produced in a skeletal muscle is a function of both the frequency of neural stimulation and the number of motor neurons involved.
The strength of contractions is controlled not only by the frequency of stimuli but also by the number of motor units involved in a contraction. A motor unit is defined as a single motor neuron and the corresponding muscle fibers it controls. Increasing the frequency of neural stimulation can increase the tension produced by a single motor unit, but this can only produce a limited amount of tension in a skeletal muscle. To produce more tension in an entire skeletal muscle, the number of motor units involved in contraction must be increased. This process is called recruitment.
All of the motor units in a muscle can be active simultaneously, producing a very powerful contraction. This cannot last for very long because of the energy requirements of muscle contraction. To prevent complete muscle fatigue, typically motor units in a given muscle are not all simultaneously active, but instead, some motor units rest, while others are active, allowing for longer muscle contractions by the muscle as a whole.
The action potentials produced by pacemaker cells in cardiac muscle are longer than those produced by motor neurons that stimulate skeletal muscle contraction. Thus, cardiac contractions are approximately ten times longer than skeletal muscle contractions. Because of long refractory periods, new action potential cannot reach a cardiac muscle cell before it has entered the relaxation phase, meaning that the sustained contractions of tetanus are impossible. If tetanus were to occur, the heart would not beat regularly, interrupting the flow of blood through the body.
Skeletal Muscle Tissue and Fiber Types
Muscle contractions are among the largest energy-consuming processes in the body, which is not surprising considering the work that muscles constantly do. Skeletal muscles move the body in obvious ways such as walking and in less noticeable ways such as facilitating respiration. The structure of muscle cells at the microscopic level allows them to convert the chemical energy found in ATP into the mechanical energy of movement. The proteins actin and myosin play large roles in producing this movement.
Skeletal Muscle Fiber Types
There are three main types of skeletal muscle fibers (cells): slow oxidative (SO), which primarily uses aerobic respiration; fast oxidative (FO), which is an intermediate between slow oxidative and fast glycolytic fibers; and fast glycolytic (FG), which primarily uses anaerobic glycolysis. Fibers are defined as slow or fast based on how quickly they contract. The primary metabolic pathway used determines whether a fiber is oxidative or glycolytic. If a fiber primarily produces ATP through aerobic pathways, it is oxidative. Glycolytic fibers primarily create ATP through anaerobic glycolysis.
Most muscles (organs) possess a mixture of each fiber (cell) type. The predominant fiber type in a muscle is determined by the primary function of the muscle. Large muscles used for powerful movements contain more fast fibers than slow fibers. As such, different muscles have different speeds and different abilities to maintain contraction over time. The proportion of these different kinds of muscle fibers will vary among different people and can change within a person with conditioning.
Skeletal Muscle Anatomy
Recall all of the structures of the fused skeletal muscle cell. If you need to, review organelles and structures specific to the skeletal muscle cells.
Structures analogous to other cell organelles:
- Sarcolemma—the membrane of the fused skeletal fiber.
- Sarcoplasm—the cytoplasm of the fused skeletal fiber.
- Sarcoplasmic reticulum—the endoplasmic reticulum of the fused skeletal fiber.
- Specialized structures in muscle cells:
- Transverse tubules (T tubules)—sarcolemma tubes filled with extracellular fluid that coordinate conduction in large muscle cells.
- Terminal cisternae—enlarged sarcoplasmic reticulum structures store calcium and surround T tubules.
- Triad—one T tubule and two terminal cisternae.

