14 Membrane Transport
Selective Permeability
The plasma membrane is the boundary of the cell; it determines what enters and exits the cell, and how the cell interacts with its environment. The cell membrane separates the extracellular and intracellular fluids, and each of these fluids contain thousands of substances. These substances often differ between the two fluids, or are at least found in very different concentrations. In order to maintain these differences, the cells need to be selectively permeable, regulating what moves in and out. Therefore cell membranes only allow some molecules through. This characteristic is why cell membranes are selectively permeable. They are not impermeable (meaning they do not prevent passage of all molecules) nor are they freely permeable (meaning they don’t let all molecules freely move across the membrane). This quality allows a cell to control what enters and exits it.
Passive and Active Passage Across (Through) the Cell Membrane
There are two categories used to describe the passage of substances through a cell membrane. They are categorized as passive (the cell does not have to use ATP), but needs some sort of driving force, and active (the cell must use ATP).
While passive processes do not use ATP, they do need some sort of driving force. This usually comes from kinetic energy in the form of a concentration gradient. Molecules will tend to move from high to low concentrations by the random movement of molecules. There are 2 main types of passive processes that occur across cell membranes.
- Diffusion and facilitated diffusion
- Osmosis and tonicity
There is also a passive process, filtration, that occurs across vessel membranes (such as capillary walls and the walls of lymphatic vessels), but it should be noted that it does not generally occur across cell membranes.
Diffusion
Diffusion relies on kinetic energy and a concentration gradient. Kinetic energy is affected by temperature, size of molecules, magnitude (steepness) of the gradient, and the medium (for example, the type of solution) the molecules are in. Anything that increases the kinetic energy of the molecules will increase the rate of diffusion.
Diffusion is the movement of molecules from an area of high concentration to an area of lower concentration. When referring to diffusion of molecules across a cell membrane, diffusion can be divided into simple diffusion and facilitated diffusion. In simple diffusion, the molecule passes directly through the phospholipid bilayer without the aid of a protein, whereas in facilitated diffusion a channel or carrier protein is used. Simple diffusion occurs with either very small or lipid soluble molecules that can pass directly through the hydrophobic region of the phospholipid bilayer of the cell membrane. Some examples of substances that use this process are oxygen (O2), carbon dioxide (CO2), and lipids. Facilitated diffusion occurs with molecules such as glucose, which may have a lower concentration inside of the cell than in the extracellular space, but that require the aid of a membrane protein for entry.
Diffusion will occur as long as there is a concentration gradient and path for movement. Once the concentration gradient no longer exists, it means that equilibrium has been reached and net diffusion ceases. This doesn’t mean that molecules still won’t move, but it does indicate that the movements are balanced in each direction and no more changes in concentration will occur.
Diffusion most commonly occurs in gasses and liquids. For example, waking up to the smell of coffee or bacon in the morning can occur because the chemicals (odorants) diffuse from the higher concentration in your kitchen to you! In the human body the diffusion of O2 and CO2 are critical for gas exchange. O2 levels are higher in your arterial blood than your tissue cells so O2 will diffuse out of the blood into your cells. CO2 has the opposite concentration gradient. CO2 levels are highest in your tissue cells (your mitochondria produce CO2 as a waste product from cellular respiration) and CO2 diffuses out of the cell into the blood. These molecules are small enough to pass through the phospholipid bilayer and therefore are examples of simple diffusion.
The following animation depicts the process of simple diffusion, showing the molecules moving from an area of high concentration to an area of low concentration. In time, the concentration gradient dissipates and equilibrium is reached.
Diffusion Across the Membrane
Molecules can be divided into four categories with regard to their ability to cross the plasma membrane. The first category is nonpolar molecules. These hydrophobic molecules can easily cross the membrane because they interact favorably with the nonpolar lipids. Note that these molecules can accumulate in the membrane because they interact so well with the lipids. The second category is small polar molecules. Although they don’t interact with the lipids, their small size allows them to pass through small temporary holes in the membrane. The third category is large polar molecules. These have difficulty crossing the membrane because of their size and poor interaction with the lipids. The last category is ionic compounds. Their charge interacts very unfavorably with the lipids, making it very difficult for them to cross the membrane.
The size, polarity, and charge of a substance will determine whether or not the substance can cross the cell membrane by diffusion. For example, even large, nonpolar substances (such as cholesterol) will freely enter the the nonpolar environment of the lipid bilayer, and some will pass through to the other side, while others will stay within the cell membrane. Small amphipathic molecules such as ethanol on the other hand, diffuse through the membrane. The lipid bilayer is much less permeable to ions because the charges are strongly excluded from the hydrophobic regions. As a general rule, charged molecules are much less permeable to the lipid bilayer.
Facilitated Diffusion
Cells must be able to move large polar and charged molecules across the lipid bilayer of the membrane in order to carry out life processes. To allow these molecules, which are not soluble in the lipid bilayer, to pass across the hydrophobic barrier it is necessary to provide ports, channels or holes through the membrane. The molecules will still move spontaneously down a concentration gradient from high to low concentration. Some of these channels can remain open at all times, allowing the molecules to move freely according to the concentration gradient. Others can be gated channels that open and close in response to the needs of the cell. In most cases these channels are very discriminatory and will only allow specific molecules to pass. The process of moving impermeable molecules across a membrane (down their concentration gradients) using channels or pores is referred to as facilitated diffusion. Because the molecules are moving down a concentration gradient, the process is driven by simple diffusion and does not require the input of additional energy from the cell.
Osmosis
Cells continually encounter changes in their external environment. Most cells have a similar blend of solutes within them, but interstitial or extracellular fluid can vary. What will happen if there is a strong concentration gradient between a cell’s interior and the fluid outside? As you know, molecules will tend to move down their concentration gradients until equilibrium is reached. You might think that solutes will flow into our out of the cell until the solute concentrations are equal across the membrane. However, not all molecules can pass through the cell membrane. The plasma membrane (lipid bilayer) is significantly less permeable to most solutes than it is to water. Therefore the WATER tends to flow in a way that establishes an equal concentration of solutes on either side of the membrane. The water flows down its own concentration gradient, with a net movement toward the region that has a higher concentration of solutes. This movement of water across a semipermeable membrane in response to an imbalance of solute is called osmosis.
The relationship between the solute concentration and amount of water is an inverse relationship. The more concentrated a solution is, the less water it contains. The fewer solutes, the more water – i.e. it is more dilute. Water follows gradients and moves from an area of more water to less water but in reality water is moved to the area with the greater number of solutes. This creates a pressure termed osmotic pressure. Cells cannot actively move water, it must follow osmotic gradients. Solutions that have a greater solute concentration will pull water via osmotic pressure. This depends on the total number of solutes, not the type. Note that some water can pass through the cell membrane but most water passes through protein membrane channels termed aquaporins.
Cells may find themselves in three different sorts of solutions. The terms isotonic, hypertonic, and hypotonic refer to the concentration of solutes outside the cell relative to the solute concentration inside the cell. In an isotonic solution, solutes and water are equally concentrated within and outside the cell. 0.9% NaCl (physiologic saline) and 5% dextrose are two common isotonic solutions used.The cell is bathed in a solution with a solute concentration that is similar to its own cytoplasm. Many medical preparations (saline solutions for nasal sprays, eye drops, and intravenous drugs) are designed to be isotonic to our cells. A hypotonic solution has a low solute concentration and a high concentration of water compared to the cell’s cytoplasm. Distilled (pure) water is the ultimate hypotonic solution. If a cell is placed in a hypotonic solution, it will tend to gain water. The solutes will “stay put” within the cell, but water molecules will diffuse such that their net flow is toward the area with a higher concentration of solutes. A hypertonic solution has a high solute concentration (lower water concentration) compared to the cell cytoplasm. Very salty or sugary solutions (brines or syrups) are hypertonic to living cells. If a cell is placed in such a solution, water tends to flow spontaneously out of the cell.
Filtration
Filtration is another passive process of moving material through a cell membrane. While diffusion and osmosis rely on concentration gradients, filtration uses a pressure gradient. Molecules will move from an area of higher pressure to an area of lower pressure. Filtration is non-specific. This means that it doesn’t sort the molecules, they pass due to pressure gradients and their size. If molecules are small enough to pass through the membrane, they will. The force that pushes the molecules is termed hydrostatic pressure.
Filtration is one of the main methods used for capillary exchange. Blood pressure provides the driving force or hydrostatic pressure to force materials out of capillaries to cells or to form the filtrate (fluid in the nephron of the kidney). Hydrostatic pressure is countered by osmotic pressure. Remember osmotic pressure is created due to increased solute concentration and will pull water toward the area of higher solutes. These two pressures must be in balance for homeostasis of fluid volumes. In our body large molecules such as plasma proteins and red blood cells should not pass out of the blood through the cell membranes lining the capillaries. If they pass through and end up in in the tissues or in the kidney and later the urine it is abnormal and a sign of disease.
Active Transport
You have just finished investigating the passive methods of transport, now let’s look at active methods. In active methods the cell must expend energy (ATP) to do the work of moving molecules. Active transport often occurs when the molecule is being moved against its concentration gradient or when moving very large molecules into our out of the cell. There are 3 main types of active processes.
- Primary Active Transport or Solute Pumps
- Endocytosis and Exocytosis
Primary Active Transport
One form of active transport involves moving ions from an area of low concentration to an area of higher concentration. If you need to roll a rock it’s much easier to roll it downhill rather than uphill, right? Active transport is moving the rock uphill and requires energy to do so. The best example of this involves the sodium (Na+)/potassium (K+) exchange pump. The interior of a cell contains a low Na+ concentration compared to the extracellular fluid. The interior of the cell (intracellular fluid or ICF) contains more K+ than the extracellular fluid (ECF). This creates an imbalance in these ions. Na+ has a concentration gradient to enter the cell, since there is more Na+ in the ECF. K+ has a concentration gradient for it to leave the cell. The cell membrane is not impermeable to these ions and some of them escape following their concentration or diffusion gradients. You will later study why it is important to maintain these ion concentrations. The Na+/K+ pump is an active transport pump that moves Na+ “uphill” back out of the cell against its concentration gradient, and at the same time moves K+ back into the cell against its concentration gradient. This pump requires ATP, a membrane protein transporter, and enzymes, to function.
Another form of active transport is termed secondary active transport. While primary active transport directly uses ATP, secondary active transport relies on the energy from electrochemical gradients to move molecules against their gradients. Primary active transport sets this up because it actively pumps ions such as Na+ out of the cell, thereby creating an electrochemical gradient for Na+ across the cell membrane. Protein transporters in the cell membrane use the energy from electrochemical gradients to transport molecules. If the molecules move in the same direction this is known as co-transport or symport. If they are moved in opposite directions this is known as counter transport or antiport. One common occurrence in the human body is the Na+/glucose transport protein known as SGLT1 which co-transports 1 glucose and 2 sodium ions into a cell. The electrochemical gradient for Na+ into the cell allows glucose to ride with it. When glucose moves into a cell, it rides the energy from the “coat tails” of the sodium ions.
Endocytosis and Exocytosis
Facilitated diffusion and active transport are not the only ways that materials can enter or leave cells. Through the processes of endocytosis and exocytosis, materials can be taken up or ejected in bulk, without passing through the cell’s plasma membrane.
In endocytosis, material is engulfed within an infolding of the plasma membrane and then brought into the cell within a cytoplasmic vesicle. To begin endocytosis, a particle encounters the cell surface and produces a dimple or pit in the membrane. The pit deepens, invaginates further, and finally pinches off to form a vesicle in the cytoplasm of the cell. Note that during the process the inside surface of the newly formed vesicle is the same as the exterior surface of the cell. Thus the integrity of the cytoplasm and the orientation of the plasma membrane are preserved. Once internalized, this new vesicle containing extracellular materials may fuse with a lysosome so that its solid contents are digested. The resulting molecules may be released to the cytoplasm for use within the cell.
There are two general forms of endocytosis: phagocytosis and pinocytosis. Phagocytosis is the uptake of large solid particles such as bacteria or cellular debris. Pinocytosis is the uptake of fluid and any small molecules dissolved within it. Cells are also capable of recognizing specific particles and engulfing them in a more targeted way, a process called receptor-mediated endocytosis. In this case, the particle first binds to a membrane protein receptor on the surface of the cell. Binding of the target particle induces the cell to engulf it.
Exocytosis is just the reverse of endocytosis. In exocytosis, an internal vesicle fuses with the plasma membrane and releases its contents to the outside. The balance of exocytosis and endocytosis preserves the size of the plasma membrane and keeps the cell’s size constant.
How are endocytosis and exocytosis important to everyday life? Immune cells protect animals by recognizing and destroying foreign objects such as bacteria. Disease-causing bacteria are recognized by proteins called receptors on the surface of the immune cell. The phagocytic immune cell will then engulf the bacterial cells (phagocytosis). The vesicle that contains these bacterial cells is called a phagosome (“phago” means “eating” and “-some” refers to “body”). The phagosome next fuses with lysosomes. Finally, the digested bacterial products are excreted through the process of exocytosis [1].
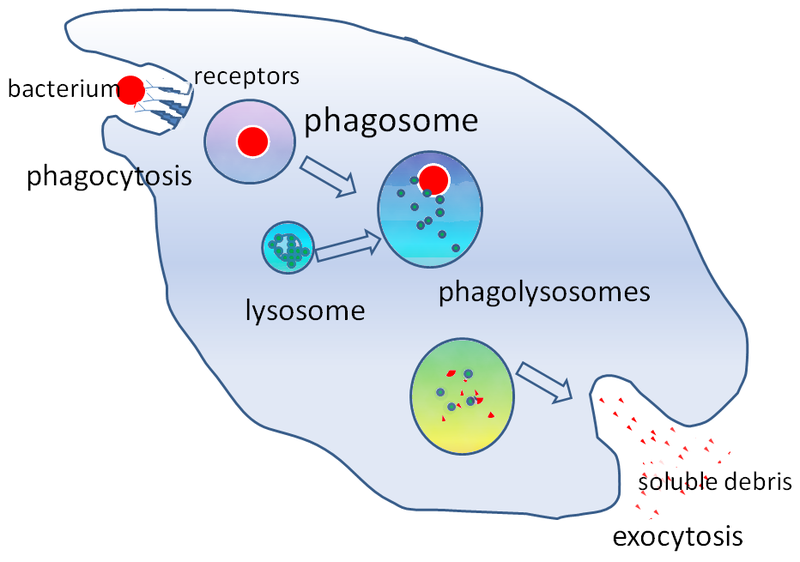
- Image: Phagocytosis of a Bacterium, Image description: A bacterium is engulfed via phagocytosis, moving into the cell within a cytoplasmic vesicle called a phagosome. Next, the phagosome will fuse with lysosomes, forming a phagolysosome for digestion of solid contents. Lastly, the digested bacterial products are excreted via exocytosis, where the vesicle will fuse with the plasma membrane to release digested bacterial products (soluble debris) outside the cell. ↵

