52 Endocrine Structures and Functions
Hormones
Hormones are the chemical messengers of the endocrine system. They elicit specific responses by binding to receptors on or in target cells.
The amount of a hormone circulating at any one time is affected by:
- the rate of hormone production
- the rate of secretion from endocrine glands
- the rate of blood flow delivering the hormone to its target cells with specific receptors
- the availability and number of hormone receptors
- the rate of biochemical degradation and elimination of the hormone
The time it takes the body to degrade 50 percent of a predetermined amount of hormone is called the hormone’s half-life. A hormone with a long half-life would persist in the blood long after the endocrine gland had stopped releasing it.
Many of these factors are a function of the type of hormone produced. Although there are many different hormones in the human body, they can be divided into three classes based on their chemical structure: lipid-derived hormones, amino acid-derived hormones, and peptide hormones.
Lipid-Derived Hormones
The lipid-derived hormones include steroid hormones and eicosanoids. Steroid hormones, the primary class of lipid hormones in humans, are derived from cholesterol and show structural similarity to that molecule. Examples of steroid hormones include estrogen and testosterone, which are released by reproductive organs, and aldosterone and cortisol, which are released by the adrenal glands.
Steroid hormones are released as synthesized. They are insoluble in water, so to circulate through the blood they bind to transport proteins in serum. Since they are bound to carrier proteins, steroid hormones typically remain in circulation longer than other classes of hormones. Recall that the “lifetime” of a molecule from production to destruction is called the half-life. The steroid hormone cortisol has a half-life of 60 to 90 minutes, whereas epinephrine, an amino acid-derived hormone, has a half-life of approximately one minute. They are eventually degraded and excreted in urine or bile.
Eicosanoids are a class of signalling molecules derived from polyunsaturated fatty acids. They are typically released as paracrine signals upon stimulation and are only active for a few seconds.
Eicosanoids are a class of signalling molecules derived from polyunsaturated fatty acids. They are typically released as paracrine signals upon stimulation and are only active for a few seconds.
Amino Acid-Derived Hormones
Of the amino acid-derived hormones, some circulate for only a few minutes while others may circulate for days. The amino acid tyrosine is the precursor for two groups of hormones: the thyroid hormones, produced in the thyroid gland; and the catecholamines (epinephrine and norepinephrine), produced in the adrenal medullae. The amino acid tryptophan is the precursor for the hormone melatonin, secreted by the pineal gland, and the hormone serotonin, which is quite widespread in the body. Some of these hormones have a circulating half-life of a few days (thyroid hormones) while some are rapidly degraded (catecholamines).
Peptide-Derived Hormones
Peptide hormones are short peptides and polypeptide chains. Recall that peptides are usually made up of fewer than 50 amino acids and proteins are typically longer than that. Some peptide hormones of longer lengths can have secondary structures. Protein hormones, being much longer than peptides, can have more extensive three-dimensional structures and can even be globular.
Peptide hormones are a diverse group and include molecules that are only a few amino acids, such as antidiuretic hormone and oxytocin (each with 9 amino acids), produced by the pituitary gland. This class also includes proteins, such as growth hormone (191 amino acids), and glycoproteins, such as follicle-stimulating hormone (92 amino acids with glycosylation). Peptide hormones can either be released as produced or stored and released in response to stimulus. Most are water soluble and are easily transported in blood plasma, but some also bind to transport proteins. Most have a half life of minutes.
Control of Hormone Production
Hormone production can be regulated by positive and negative feedback pathways. In positive feedback systems, the release of a hormone leads to an action that stimulates release of more of the same hormone.
Example: Positive Feedback Loop in the Endocrine System
Oxytocin released by the pituitary gland prior to childbirth stimulates contraction of the uterus and increases pressure on the cervix. The increased pressure signals the pituitary to release even more oxytocin, which increases force of contractions, leading to even more cervical pressure. This amplification cycle continues until childbirth is complete. After the baby is born, the stretch receptors are no longer stimulated and the release of oxytocin decreases.
In a hormonal negative feedback loop, when a stimulus causes the release of a hormone (Hormone A), the hormone binds to the target cell receptor, causing the necessary metabolic change toward homeostasis. In a negative loop, the effect of this metabolic change is to counter the stimulus that caused the release of Hormone A. Once the cause of the stimulus returns to normal range, the production of that hormone stops and plasma level of that hormone returns to the normal (pre-stimulus) level. In this way, the concentration of most hormones in blood is maintained within a narrow range.
Example: Negative Feedback Loop in the Endocrine System
The hypothalamus monitors the plasma level of thyroid hormones, among others. When the level drops, the hypothalamus stimulates the anterior pituitary to release a hormone (Thyroid Stimulating Hormone, or Thyrotropin) that stimulates the thyroid to release thyroid hormones. Increased levels of the thyroid hormones in the blood then feed back to the hypothalamus and anterior pituitary to inhibit further stimulation of the thyroid gland. Other homeostatic imbalances, such as low body temperature, can also stimulate the hypothalamus to stimulate the anterior pituitary to release thyrotropin. Thyroid hormones play an important role in metabolic heat production via ATP production.
Stimuli Regulating Hormone Production
There are three mechanisms by which endocrine glands are stimulated to synthesize and release hormones: humoral regulation, hormonal regulation, and neural regulation.
Humoral Stimuli
The term humoral is derived from the term humor, which refers to bodily fluids such as blood and other extracellular fluids. Humoral stimuli regulate the release of hormones in response to specific changes in extracellular fluids, such as the concentration of a particular ion or solute in the blood or even the overall solute levels in the blood.
Example: Blood Glucose Level Rise Triggers Pancreatic Release of Insulin
Tropic Hormonal Stimuli
With tropic hormonal stimuli, a hormone is produced and released by an endocrine gland in response to another hormone (known as “tropic hormones”). These hormones controlling release of another hormone are called tropic (meaning “turn toward,” pronounced “tro’-pick”; not same as geographical “trop-ic”).
Example: Hypothalamus and Hormones
The hypothalamus produces hormones that stimulate the anterior pituitary. The anterior pituitary in turn releases hormones that regulate hormone production by other endocrine glands. For example, the anterior pituitary releases thyroid-stimulating hormone, which stimulates the thyroid gland to produce the hormones T3 and T4. As blood concentrations of T3 and T4 rise, they inhibit further hormone production by both the pituitary and the hypothalamus in a negative feedback loop.
Note… Sometimes students get confused between tropic and trophic hormones. Tropic hormones stimulate release of other hormones from endocrine cells, such as those discussed here. Trophic (meaning “nourishment or nurse”) hormones stimulate non-endocrine cell growth and development, such as growth hormone, estrogen and testosterone.
Neural Stimuli
The nervous system can also directly stimulate endocrine glands to release hormones through a mechanism known as neural stimuli.
Example: Neuronal Signaling from Sympathetic Nervous System
Neuronal signaling from the sympathetic nervous system directly stimulates the adrenal medulla to release the hormones epinephrine and norepinephrine in response to stress.
Distance of Hormonal Signaling
Cells communicate with one another via chemical messengers. The communication may happen between cells close by or far away from the cells that produces the messenger (signal). For example, released hormones travel throughout the body and affect any cells with receptors for the specific hormones. Autocrine signaling (auto- means self) affects the cells that released the signaling molecule. Paracrine signaling (para- means alongside) affects local cells other than the secreting cells. While traditionally a hormone is thought to have its effect at a distance from where it is secreted, the definition of hormones now include paracrine and autocrine mechanisms as well. The all-inclusive term, Endocrine signaling, includes all types of communication where chemical molecules produced from a cell affect the metabolism of another cell (paracrine or endocrine) or that of its own (autocrine).
Hormone Receptors
Hormones mediate changes in cells by binding to specific receptors; individual hormones have their own unique receptor or class of receptors. A cell that has a receptor for a specific hormone is called a target cell. A hormone can, therefore circulate throughout the body, contacting many different cell types, but affecting only those cells that possess the specific receptor. A receptor is a protein (cell membrane or intracellular) that binds specifically to a particular hormone. Receptors for a specific hormone may be found in or on many different target cells, or may be limited to a small number of specialized cells.
Example: Thyroid Hormones
The number of receptors that respond to a hormone determine the cell’s potential sensitivity to that hormone and the resulting cellular response. The number of receptors that respond to a hormone can change, resulting in increased or decreased cell sensitivity. Since alcohol (ethanol) inhibits glutamate receptor channels, people with high alcohol levels show higher number of glutamate receptors in a classic example of up-regulation that makes the cells more sensitive to the hormone to try to maintain the normal level of response to glutamate (homeostasis). The number of cell surface receptors can also decrease in response to rising hormone levels, called down-regulation, leading to reduced cellular response to the hormone. Thus, the action of a hormone can be regulated either by the amount of hormone in circulation or by the number of receptors (either inside the cell or on the cell surface) available at that time for that hormone.
Hormones bind to receptors and cause changes in cellular function. Receptors can be found either inside the cell (intracellular receptors) or on the cell’s surface (plasma membrane receptors). Knowing the location of a specific receptor provides important information about the mechanism that specific hormone uses to affect cell physiology.
Intracellular Hormone Receptors
Steroid hormones are lipophilic and need transport proteins in the blood. Once released from their transport protein, the non-polar hormone is able to diffuse across the plasma membrane of cells. Recall that the lipid bilayer of the plasma membrane of cells uses amphiphilic phospholipids to compartmentalize the cytoplasm of a cell. When a steroid hormone crosses the plasma membrane of a target cell, it binds to an intracellular hormone receptor in the cytoplasm, on intracellular membrane system (ER) or, within the nucleus of the cell. The receptor/hormone complex can then bind to a specific site on DNA and act as a transcription regulator to increase or decrease the synthesis of particular mRNA molecules coded by these specific genes. This, in turn, alters mRNA production, which determines the amount of corresponding protein that is synthesized. The steroid hormone regulates specific cell processes. The rate of transcription and protein synthesis is directly proportional to the amount of hormone forming receptor/hormone complexes; so if the hormone production increases, so does the physiological effect in the body.
The thyroid hormones, T3 and T4, also use plasma transport proteins and intracellular DNA-binding receptors. There are, however, some important physiological differences with the steroid hormone mechanism. The target cells have membrane transport proteins that transport the thyroid hormones into the cell. The Thyroid hormone receptor is found bound to a transcription repressor protein on the DNA. The binding of the hormone with the receptor-repressor complex to form the receptor/hormone complex causes the repressor protein to dissociate, and a transcription activator protein becomes associated with the receptor. This, in turn, initiates transcription.
Plasma Membrane Hormone Receptors
Most peptide and amino acid hormones are polar and therefore cannot diffuse through the plasma membrane of cells. So, they bind to plasma membrane hormone receptors on the outer surface of the plasma membrane. Unlike steroid hormones, polar hormones also alter intracellular processes and can also affect the target cell’s transcription. Since they cannot enter the cell and act directly on any DNA-binding proteins, they exert their transcriptional effects through intermediate molecules called second messengers as described below. Catecholamines (amine class) and polar eicosanoids (lipid-derived class) also bind to cell-surface hormone receptors.
Binding of these hormones to a cell membrane surface receptor results in activation of a signaling pathway that triggers a cascade of intracellular activity and specific effects associated with the hormone. Most hormones that bind at the surface receptor remain outside the target cell. Some hormones are taken into the cell by endocytosis to initiate the intracellular biochemical response from within vesicles. The hormone that initiates the signaling pathway, the first messenger, activates a second messenger within the cell.
Example: Blood Sugar and Glucagon
G-protein-coupled Receptors
G-proteins are a class of trans-membrane cell surface proteins that can be activated by hormones or ions and other chemicals for cell signaling. G-proteins remain inactive unless a hormone is bound to its cell surface receptor. Inactive G-proteins are bound to GDP on its cytoplasmic side. When a hormone binds to the cell surface receptor the bound GDP is replaced by GTP.
Activated G-proteins can have different functions depending on the hormone receptor: it can open a membrane protein channel; it can release a small molecule; or it can activate a membrane-bound enzyme. There is a large variety of G-protein-induced effects. For example, G-protein-linked ion channels can stimulate movements of ions across the membranes. There are specific channels for potassium, sodium, calcium or chloride.
For G-protein activated membrane-bound enzymes, there are large numbers of activated enzymes. One of the membrane bound enzymes that the G-Protein coupled receptor (GPCR) activates upon binding to a hormone molecule is the adenylate cyclase. This enzyme catalyzes the conversion of ATP to cyclic AMP (cAMP), which, in turn, activates a class of enzymes called protein kinases. These kinases transfer a phosphate group from ATP to a substrate molecule in a process called phosphorylation. The phosphorylation of a substrate molecule changes its shape, thereby activating it. This chain of reactions, where hormone binding to the GPCR leads to the appearance of cAMP in the cytoplasm as the second messenger which, in turn, leads to the activation of protein kinase is an example of a “reaction cascade.” In this case, since a signal from the exterior of the cell is transferred to the interior via the formation of a “second messenger,” the process is called “signal transduction cascade.” Other second messengers that can be involved as a result of hormone binding to a cell membrane receptor include cyclic GMP (derived from guanosine triphosphate), tyrosine kinases, inositol phospholipids, and even calcium ions. Cellular responses to hormone binding of a cell membrane receptor include altering membrane permeability, activating metabolic pathways, stimulating synthesis of proteins and enzymes, and hormone release.
The binding of a hormone at a single cell membrane receptor causes the activation of many G-proteins, which can catalyze many reactions simultaneously. Thus, the effect of a peptide hormone is amplified as the signaling cascade progresses. A small amount of hormone can trigger the formation of a large amount of cellular product. To stop hormone activity, the cascading chemical reaction is interrupted; for example cAMP is deactivated by the cytoplasmic enzyme phosphodiesterase (PDE). PDE is always present in the cell, and it breaks down cAMP spontaneously, preventing overproduction of cellular products. The specific response of a cell to a lipid-insoluble hormone depends on the type of receptors that are present on the cell membrane and the substrate molecules present in the cell cytoplasm.
Endocrine Glands
The Pituitary Gland and Hypothalamus
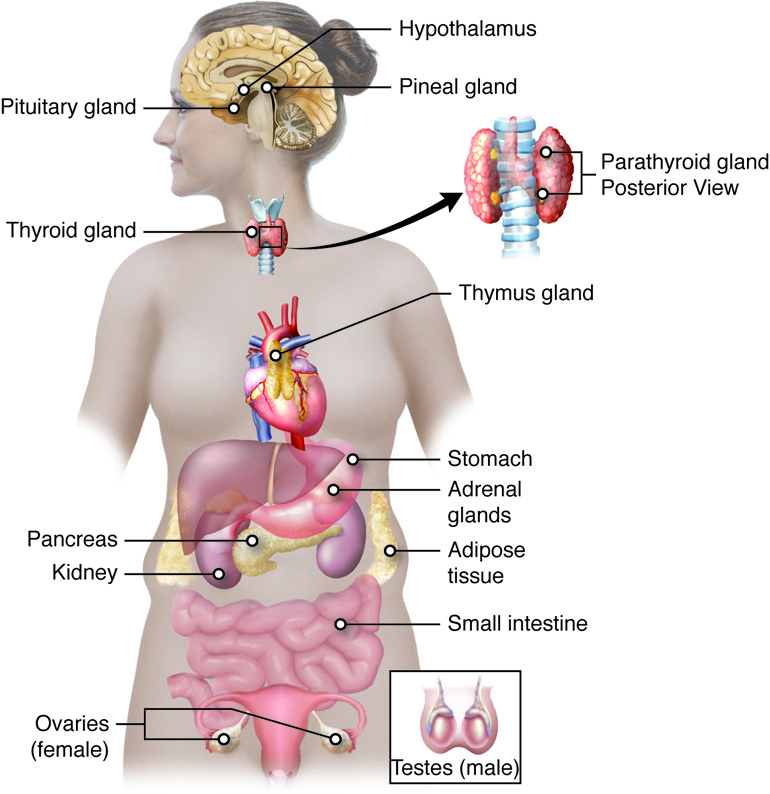
The pituitary-hypothalamus axis links the nervous system with the endocrine system. The hypothalamus is a region of the brain that is located inferior to the thalamus. It coordinates signals from internal organs and other regions of the brain and regulates a response from the endocrine system via the pituitary. The hypothalamus secretes both releasing hormones that stimulate the anterior pituitary to secrete a hormone and inhibiting hormones that inhibit the release of a hormone from the anterior pituitary.
The pituitary gland is a pea-sized gland located at the base of the brain attached to the hypothalamus via a stalk called the infundibulum. The pituitary gland is primarily regulated by nerve impulses or hormones released by neurosecretory cells of the hypothalamus. The pituitary, in turn, releases hormones that either have a direct effect on target cells or regulate hormone production by other endocrine glands. Those hormones that control the release of another hormone from an endocrine gland are called tropic hormones. The pituitary has two distinct regions: the anterior pituitary, and the posterior pituitary.
The anterior pituitary secretes seven different peptide or protein hormones: growth hormone(GH), prolactin (PRL), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ATCH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and melanocyte stimulating hormone (MSH). The posterior pituitary is an extension of the brain and releases hormones produced by the hypothalamus. The posterior pituitary releases antidiuretic hormone (ADH) (also known as vasopressin) and oxytocin. The pituitary looks like one gland because the anterior and posterior pituitary do not have externally visible distinctions, but from a cell and tissue perspective, they are really two different and distinct organs.
Anterior Pituitary
The anterior pituitary gland, or adenohypophysis, is surrounded by a capillary network. This capillary network is a part of the hypophyseal portal system that carries substances from the hypothalamus directly to the anterior pituitary. Remember that substances such as hormones can only leave or enter the circulatory system at capillaries, and portal systems (portal systems are also found in the digestive system) move material from one capillary bed to another without returning it to the main circulation. Anterior pituitary hormones then enter the capillaries and travel to the heart and through the systemic system in the same way other hormones do.
Several anterior pituitary hormones (TSH, ACTH) are tropic hormones, because they control the functioning of other endocrine glands. While these hormones are produced by the anterior pituitary, their production is controlled by regulatory hormones produced by the hypothalamus. Negative feedback mechanisms regulate how much of these regulatory hormones is released, and how much anterior pituitary hormone is secreted.
Posterior Pituitary
The posterior pituitary is significantly different in structure and function from the anterior pituitary. As its name implies, the posterior pituitary is behind the anterior pituitary (toward the back). It contains mostly axons of secretory neurons and neuroglial cells; the cell bodies of these neurons are in the hypothalamus. The posterior pituitary and the infundibulum together are referred to as the neurohypophysis.
The posterior pituitary does not produce hormones, but stores hormones produced by the hypothalamus and releases them into the bloodstream. The hormones antidiuretic hormone (ADH) and oxytocin are produced by neurons in the hypothalamus and transported within these axons along the infundibulum to the posterior pituitary. They are released into the posterior pituitary capillaries in response to neural signaling from the hypothalamus. These hormones are considered to be posterior pituitary hormones, even though they are produced by the hypothalamus, because that is where they are released into the circulatory system.
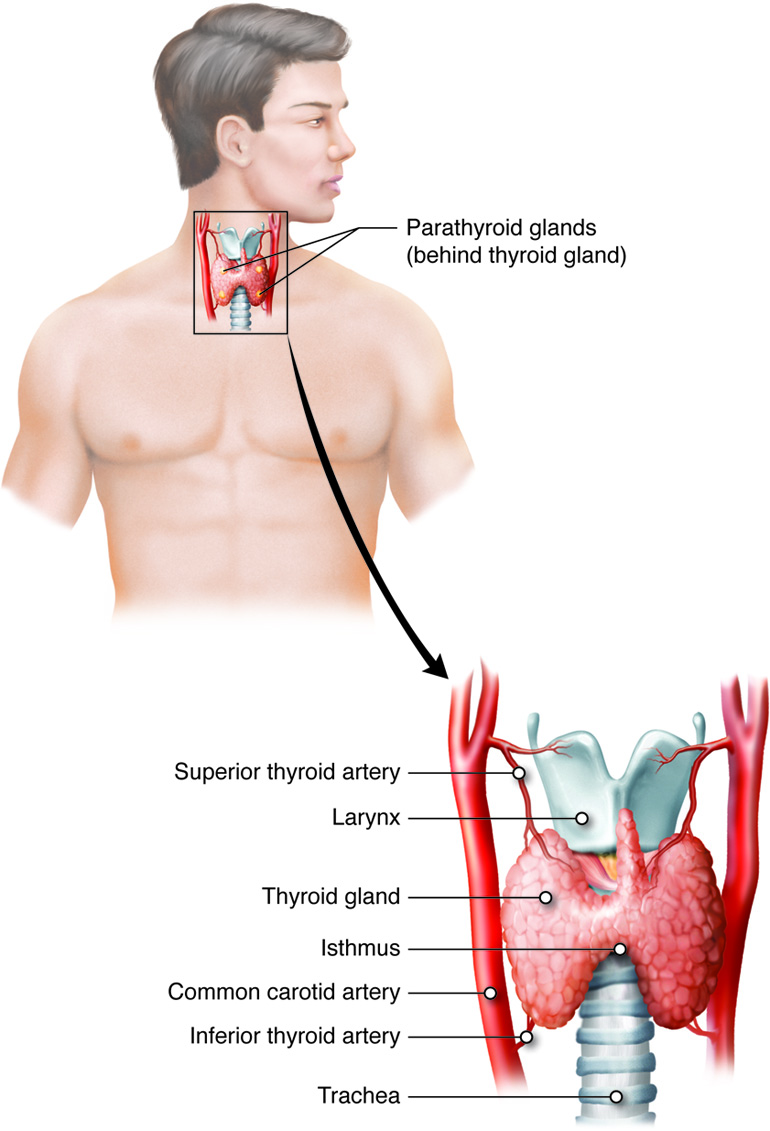
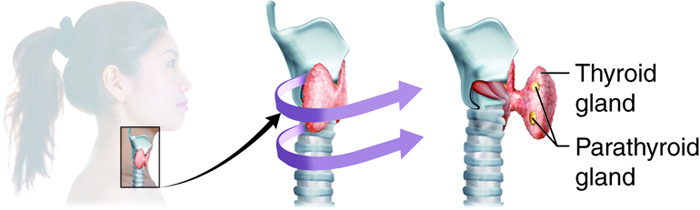
Thyroid Gland
The thyroid gland possesses two lobes that are connected by the isthmus. It is located in the neck, just below the larynx with the isthmus in front of and lobes lateral to the trachea. It has a dark red color due to its extensive vasculature. When the thyroid increases in size due to dysfunction, it can be felt under the skin of the neck. The main function of the thyroid gland is the synthesis and storage of thyroid hormones that are involved in maintaining metabolic homeostasis.
The thyroid gland is made up of many spherical thyroid follicles, which are lined with simple cuboidal epithelium. These follicles contain a viscous fluid called colloid that stores the glycoprotein thyroglobulin, the precursor to the two thyroid hormones. Thyroglobulin is not normally released into circulation unless the thyroid gland is damaged due to disease or injury. Other endocrine cells, called parafollicular cells, are located between adjacent follicles and produce a different hormone, calcitonin, that is involved in blood calcium homeostasis.
Synthesis of Thyroid Hormones
Unlike most endocrine glands, the thyroid gland stores large amounts of some of the hormones it synthesizes. Thyroglobulin is produced and secreted by follicle cells into the lumen of follicles as a colloid. There it undergoes post-translational modification to produce functioning thyroid hormones. Iodide molecules are added to the thyroglobulin precursor to produce the hormones thyroxine and triiodothyronine. Thyroxine is also known as T4 because it contains four atoms of iodine, and triiodothyronine is also known as T3 because it contains three atoms of iodine. In developed countries, the iodide required for hormone synthesis is obtained primarily from iodized salt. However, seafood and plants grown in iodine rich soil at lower elevations also provide the required iodide. Since iodine as a molecule is quite volatile, the food grown in higher elevations (lower atmospheric pressure) lacks sufficient iodine. Iodide ions are actively transported into the follicular lumen from the capillaries by follicle cells. Follicle cells are stimulated to release stored T3 and T4 from the lumen into the blood capillaries by thyroid stimulating hormone (TSH), which is produced by the anterior pituitary. Follicle cells also begin synthesizing more T3 and T4 in response to TSH stimulation.
A third hormone, calcitonin, is produced by parafollicular cells, or C cells of the thyroid. Calcitonin release is not controlled by TSH, but instead is released when calcium ion concentrations in the blood rise. Calcium ions bind to specific receptor on the C cell and stimulate release of calcitonin. Calcitonin acts primarily in children to lower blood calcium levels when levels get too high. Calcitonin causes decreased tubular reabsorption of Ca2+ in the kidneys, leading to calcium loss in urine. It inhibits bone resorption activity of osteoclasts and calcium absorption in intestine to also reduce plasma calcium levels. It is also suspected to have an indirect effect stimulating osteoblast activity and development. However, in adult humans it appears to play only a minor role in calcium homeostasis because abnormalities in calcitonin production do not appear to be associated with specific plasma calcium imbalances. Research has implicated its role during times of high calcium demands, such as pregnancy and lactation.
Parathyroid Glands
The four parathyroid glands are each the size of a grain of rice and are usually located on the posterior surface of the thyroid gland. The exact location and number of parathyroid glands can vary from person to person. The parathyroid glands are named for their proximity to the thyroid gland (para- means next to), often seeming to be part of the same gland; however, their cells are distinct from those of the thyroid gland.
Each parathyroid gland is covered by connective tissue and contains many secretory cells called chief cells, which synthesize and secrete parathyroid hormone (PTH). Another type of cell, oxyphil cells, can be distinguished histologically in the parathyroid but they are not clearly understood. They appear to increase during renal failure, but their function is still a subject of clinical research.
PTH is synthesized as a pro-peptide that is cleaved into an active hormone, which is vital in maintaining blood calcium levels. Calcium is required for nerve impulse transmission, for muscle contractions and for many cellular processes–including signal transduction. Therefore, plasma calcium concentrations must be maintained within a narrow normal range of 9–10 mg/dL. PTH functions by increasing blood calcium concentrations when calcium ion levels fall below normal. Calcium ions bind to specific receptors on the chief cell and inhibit release of PTH; when the plasma calcium level falls, PTH secretion is stimulated. PTH stimulates reabsorption of calcium from filtrate during urine formation, increased absorption in the intestines from digested products and increased activity of osteoclasts to release calcium (and phosphate) from bone matrix into the plasma. There is some evidence that it may also indirectly inhibit osteoblast activity.
A parathyroid adenoma is a benign tumor of the parathyroid gland that can cause an overproduction of PTH leading to hyperparathyroidism. Hyperparathyroidism results in hypercalcemia, which can lead to kidney stones. Additionally, increased rate of bone resorption due to higher osteoclast activity due to this condition may also lead to osteoporosis. Usually only one of the four parathyroid glands is affected and can be surgically removed without any adverse effects. However, removal of all the parathyroid glands will cause an imbalance in blood calcium levels resulting in death.
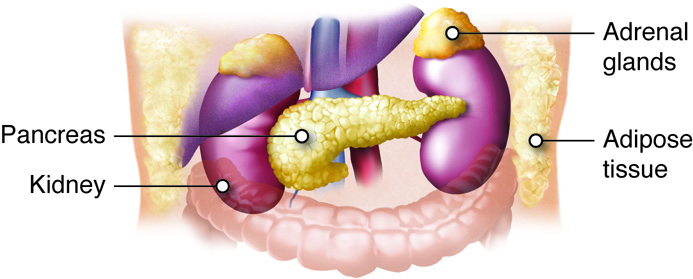
Adrenal Glands
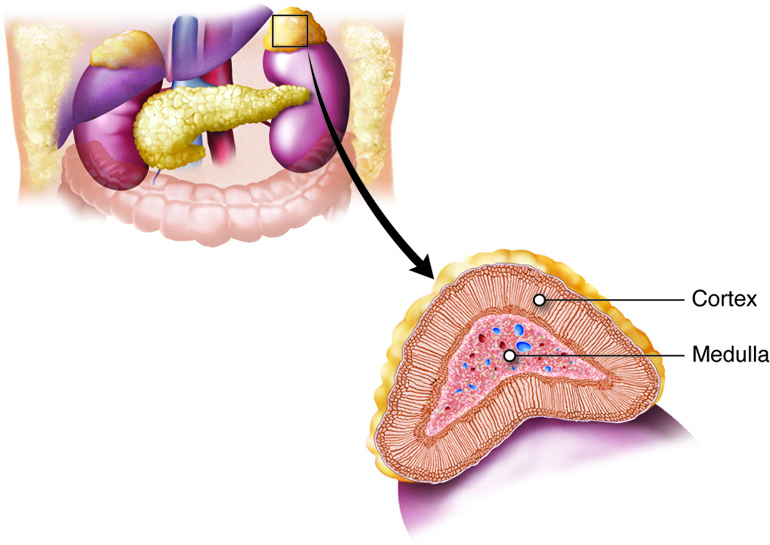
The adrenal glands help regulate the body’s response to stress, controlling blood pressure, and maintaining the body’s water, sodium, and potassium levels. The adrenal glands are associated with the pair of kidneys which are retroperitoneal and lateral to the spinal column ; one gland is located on the superior surface of each kidney (hence they are also known as suprarenal glands). The adrenal glands consist of an outer adrenal cortex (cortex means outer layer) and an inner adrenal medulla (medulla means middle). Functionally and anatomically the adrenal gland is really two glands packaged together. The cells of the cortex are endocrine and those of the medulla are neurosecretory. The cells look very different and embryologically they come from different tissues. These regions secrete different hormones: the adrenal cortex produces steroid hormones, and the adrenal medulla produces catecholamine hormones.
The adrenal glands have a large blood supply. They receive arterial blood from the renal arteries, the phrenic arteries, and suprarenal arteries from the aorta. Arterial blood enters the adrenal glands at the adrenal cortex and drains into venules in the adrenal medulla. The suprarenal vein of the right adrenal gland drains into the inferior vena cava, and the suprarenal vein of the left adrenal gland drains into the left renal vein.
Adrenal Cortex
The adrenal cortex is the outer layer of the adrenal gland and is made up of layers of epithelial cells and associated capillary networks. These layers form three distinct regions that secrete different steroid hormones: the outer zona glomerulosa produces mineralocorticoids (influence salt and water balance), the middle zona fasciculata produces glucocorticoids (impact metabolism and inflammation), and the inner zona reticularis produces androgens (regulate catabolism and sexual characteristics).
The main mineralocorticoid is aldosterone, which regulates the concentration of ions in urine, sweat, and saliva. Aldosterone release from the adrenal cortex can be triggered by a number of things including decrease in blood concentrations of sodium ions, blood volume, or blood pressure, or an increase in blood potassium levels.
The three main glucocorticoids are cortisol, corticosterone, and cortisone. The glucocorticoids stimulate the synthesis of glucose from non-glycogen sources, and they promote the release of fatty acids from adipose tissue. These hormones increase blood glucose levels to maintain levels within a normal range between meals. They are secreted in response to ACTH, and levels are regulated by negative feedback.
Androgens are a class of hormones and that affect sexual characteristics. Testosterone is associated with male sexual characteristics, while progesterone and estrogen are associated with female reproductive function; androstenedione is an intermediate molecule in the synthetic pathways of these and many of the steroid hormones.
Adrenal Medulla
The adrenal medulla is the inner layer of the adrenal glands and contains chromaffin cells, which are large, irregularly shaped cells that are closely associated with blood vessels. These cells are innervated by autonomic (involuntary) nerve fibers from the central nervous system, which allows for quick hormone release.
Chromaffin cells of the adrenal medulla produce epinephrine (adrenaline) and norepinephrine (noradrenaline). Epinephrine is the primary adrenal medulla hormone accounting for 75–80 percent of its secretions. Epinephrine and norepinephrine increase heart rate, breathing rate, cardiac muscle contractions, and blood glucose levels. They also accelerate the breakdown of glucose in skeletal muscles and stored fats in adipose tissue.
Both are stored in vesicles or granules in the adrenal medulla, very similar to the way posterior pituitary cells store the neurosecretory hormones from hypothalamus for release. The release of epinephrine and norepinephrine into the blood is stimulated by neural impulses from the sympathetic nervous system. These neural impulses originate from the hypothalamus in response to stress to prepare the body for the fight-or-flight response.
Pineal Gland
The pineal gland is located between the cerebral hemispheres of the brain. It is attached to the roof of the third ventricle in the diencephalon. The pineal gland consists of secretory cells, called pinealocytes, that secrete the hormone melatonin. Melatonin is derived from serotonin (a neuropeptide) and is regulated in response to the light and dark of the environment (the diurnal cycle). Photons, packets of light, are detected by the retinas of the eyes, which initiate a nerve impulse that is detected by the pineal gland. This mechanism is similar to the process in the posterior pituitary or adrenal medulla. The pineal gland synthesizes the highest levels of melatonin during the night, when light levels are the lowest, and the increased blood concentrations of melatonin makes us sleepy. Blood concentrations of melatonin are lowest during the day, when light exposure inhibits the synthesis of the hormone.
The target cells of melatonin are located in the suprachiasmatic nucleus (SCN) of the brain. The SCN functions as a biological clock that regulates physiological processes such as the sleep-wake cycle, appetite, and body temperature. Melatonin is thought to inhibit the release of gonadotropins from the anterior pituitary, which affect the onset of puberty.
Higher melatonin levels at night make us sleepy, and a disruption in melatonin synthesis can disrupt the sleep cycle. Travel across several time zones can result in disruptions to the sleep cycle, or jet lag. This is due to the change in the dark-light cycle that the body is accustomed to, and it can take several days for melatonin synthesis to adapt to the change. Melatonin supplements are available to treat jet lag as well as other sleep disorders; however, their efficacy has not been conclusively established.
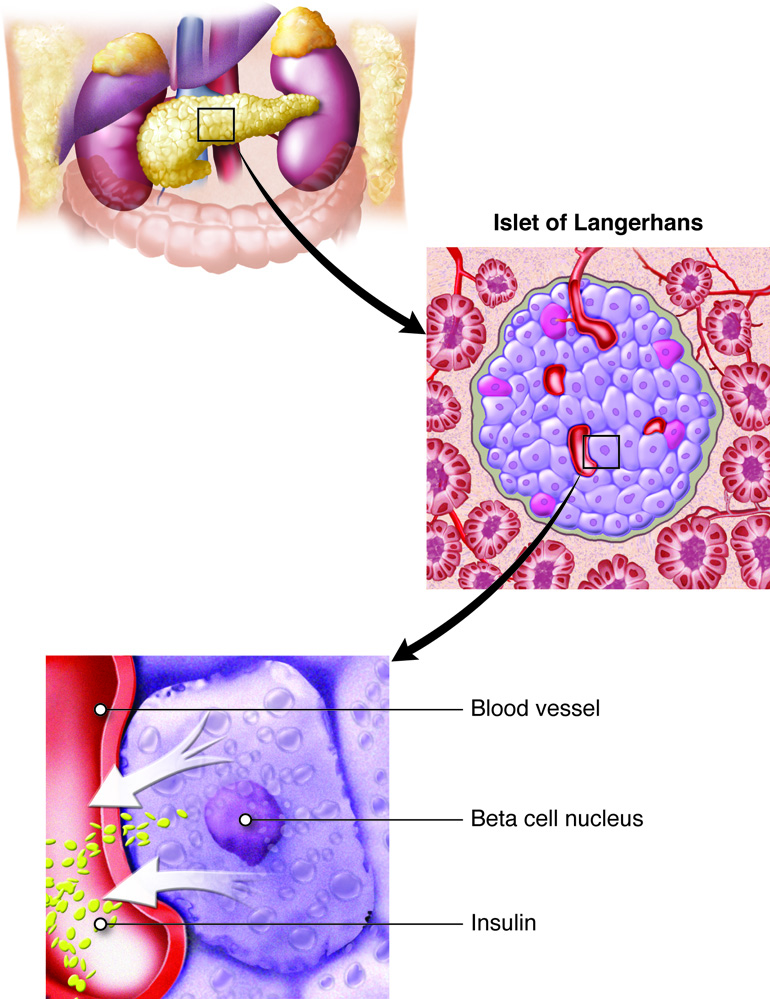
The Pancreas
The pancreas is an elongated organ that plays a central role in energy metabolism, storage, and utilization of glucose (carbohydrate). It is located slightly dorsal to the stomach and between the stomach and the small intestine. The tapered distal end lies in contact with the spleen, while the ducts from broader proximal end enter the duodenum at gastro-duodenal junction. The pancreas contains both exocrine cells that excrete digestive enzymes and endocrine cells that release hormones. Approximately 99 percent of pancreatic cells are exocrine cells that are arranged around ducts in clusters called acini (singular is acinus).
The endocrine cells of the pancreas form clusters called pancreatic islets or the islets of Langerhans (islets are small islands) with associated blood capillaries. The pancreatic islets contain two major cell types: alpha cells, which constitute 20 percent of the total mass of the islets and produce the hormone glucagon, and beta cells, which constitute 75 percent of the total mass of the islets and produce the hormone insulin. These hormones regulate blood glucose levels. Alpha cells release glucagon as blood glucose levels decline below the set point. When blood glucose levels rise, alpha cells stop secreting glucagon, and beta cells then release insulin. When blood glucose levels drop below the set point (which does not happen under normal conditions), beta cells stop secreting insulin.
Pancreatic cells also contain two minor populations of cells: delta cells, which constitute 4 percent of the total mass of the islets and secrete somatostatin, and F (or PP) cells, which constitute 1 percent of the total mass of the islets and secrete pancreatic polypeptide. They have specific paracrine regulator effects in the pancreas. Pancreatic polypeptide is secreted after a high protein meal or fasting and inhibits pancreatic exocrine secretion and stimulates gastric juice secretion (opposite effect of cholecystokinin, CCK, of the small intestine). It also stimulates both alpha and beta cells.
Gonads
The gonads, the male testes (sing. testis) and female ovaries, function in production of gametes (sperm and ovum) and also produce steroid hormones. The testes produce androgens, testosterone being the most prominent. Testosterone stimulates the development of male secondary sex characteristics and the production of sperm cells. The testes also produce the hormone inhibin, which inhibits the release of the tropic hormone from the anterior pituitary follicle-stimulating hormone (FSH) needed for the development of sperm (spermatogenesis).
The ovaries produce the hormones estrogen and progesterone, which stimulate the development of female secondary sex characteristics, regulate the menstrual cycle, and prepare the body for childbirth. The ovaries also produce the hormone inhibin, which inhibits the release of FSH.
The placenta, which supplies the necessary nutrients to the fetus, is also an endocrine organ. It synthesizes and secretes a number of hormones that are crucial for the maintenance of pregnancy. The human chorionic gonadotropin hormone (hCG), that is the basis of urine pregnancy tests is another of the placental hormones. As the fetus develops, the placenta takes over the production of estrogen and progesterone to maintain pregnancy. The high level of estrogen facilitates the growth of the uterus and the mammary glands during gestation. Progesterone is important in suppressing maternal immune response towards the fetus and inhibiting uterine smooth muscle contraction. The placenta also produces relaxin, that affects collagen metabolism and softens the pubic symphysis to facilitate birthing. It also produces lactogen, which is structurally similar to prolactin and growth hormone (pituitary hormones), but its role, if any, in human lactation is still being investigated.
Review of the Endocrine Glands and Hormones

| Endocrine Gland | Associated Hormones | Main Effect |
|---|---|---|
| Pituitary (Anterior) | Growth hormone | Promotes growth of body tissues |
| Prolactin | Promotes milk production | |
| Thyroid-stimulating hormone (TSH) | Stimulates thyroid hormone release | |
| Adrenocorticotropic hormone (ACTH) | Stimulates hormone release by adrenal cortex | |
| Follicle-stimulating hormone (FSH) | Stimulates gamete production | |
| Luteinizing hormone (LH) | Stimulates androgen/estrogen production by gonads | |
| Pituitary (Posterior) | Antidiuretic hormone (ADH; also called vasopressin) | Stimulates water reabsorption by kidneys |
| Oxytocin | Stimulates uterine contractions during childbirth | |
| Thyroid | Thyroxine, triiodothyronine | Stimulate metabolism |
| Calcitonin | Reduces blood Ca2+ levels, primarily in children | |
| Parathyroid hormone (PTH) | Increases blood Ca2+ levels | |
| Adrenal (Cortex) | Aldosterone | Increases blood Na+ levels, and related water conservation by kidneys, Decreases blood K+ levels |
| Cortisol, corticosterone, cortisone | Increase blood glucose levels | |
| Adrenal (Medulla) | Epinephrine, norepinephrine | Stimulate fight-or-flight response |
| Pineal | Melatonin | Regulates sleep cycles |
| Pancreas | Glucagon | Increases blood glucose levels |
| Insulin | Decreases blood glucose levels | |
| Testes | Testosterone | Stimulates development of male secondary sex characteristics and sperm production |
| Inhibin | Inhibits secretion of FSH | |
| Ovaries | Estrogen and progesterone | Stimulates development of female secondary sex characteristics, egg production and preparation of the body for childbirth |
| Inhibin | Inhibits secretion of FSH |
Organs with Secondary Endocrine Function
There are several organs whose primary functions are non-endocrine but that also possess endocrine functions. These include the heart, gastrointestinal tract, kidneys, adipose tissue, skin, and thymus.
Heart and Cardiovascular System
The heart possesses specialized cardiac muscle cells, which are endocrine cells in the walls of the atria. These cells respond to increased blood volume by releasing the hormone atrial natriuretic peptide (ANP). Natrium is the name for sodium in many languages and is the reason that Na is the chemical symbol for sodium. High blood volume causes the cells to be stretched, opening stretch-activated membrane channels, resulting in hormone release. ANP acts on the kidneys to reduce the reabsorption of Na+, causing Na+ and water to be excreted in the urine. ANP also reduces the amounts of renin released by the kidneys and aldosterone released by the adrenal cortex, further preventing the retention of water. In this way, ANP reduces the concentration of Na+ in the blood and causes a reduction in blood volume and blood pressure. Another natriuretic hormone, BNP (misnamed brain natriuretic because it was first isolated from pig brains) from the heart ventricle, enhances the effect of ANP.
Endothelial cells lining the cardiovascular system also have an endocrine function. They produce paracrine endothelin, a vasodilator and stimulator of ANP secretion, and nitric oxide (NO), a vasodilator and inhibitor of ANP secretion.
Digestive System
The digestive system produces several hormones that aid in digestive and metabolic homeostasis. Endocrine cells are located in the mucosa of the GI tract throughout the stomach and small intestine. Hormone secretion is controlled by receptors monitoring the chemical content of the digestive lumen. Some of the hormones produced include gastrin (from stomach), secretin, and cholecystokinin CCK (from small intestine) that act on the GI tract and accessory organs such as the pancreas, gallbladder, and liver. They trigger the release of digestive juices that help to break down and digest food in the GI tract. The GI tract also produces the hormones glucose-dependent insulinotropic peptide (GIP) (from stomach) and glucagon-like peptide 1 (GLP-1) (from small intestine). These hormones are secreted in response to glucose in the intestinal lumen. GIP and GLP-1 target cells are beta cells in the pancreas, which are stimulated to release insulin and alpha cells that are inhibited from releasing glucagon. The stomach also produces ghrelin that mediates hunger, stimulating appetite and growth hormone release.
The liver, as an accessory organ of the digestive system, also has an endocrine function in production of insulin-like growth factor (IGFs) and thrombopoietin (THPO). IGFs work with growth hormone to regulate cell metabolism while THPO triggers the formation of platelets in the blood. The liver also produces prohormones (angiotensinogen and calcidiol, vitamin D) and plasma proteins that transport many of the hormones.
Kidneys and Urinary System
The adrenal glands associated with the kidneys are major endocrine glands, and the kidneys themselves also possess endocrine functions. Renin (‘renal’ generally describes aspects of the kidney) is released in response to decreased blood volume or pressure and is part of the renin-angiotensin system that is responsible for the formation of angiotensin II and ultimately leads to the release of aldosterone. Both angiotensin II and aldosterone then causes the retention of Na+ and water, raising blood volume. The kidneys also release the steroid hormone calcitriol, which is the biologically active form of vitamin D that aids in the absorption of Ca2+. Erythropoietin (EPO) is a protein hormone that triggers the formation of red blood cells in the bone marrow. EPO is released in response to low oxygen levels. Because red blood cells are oxygen carriers, increased production results in greater oxygen delivery throughout the body. The banned substance EPO had been used by athletes at one point to improve performance, as greater oxygen delivery to muscle cells allows for greater endurance. Because red blood cells increase the viscosity of blood, artificially high levels can cause severe health risks.
Adipose Tissue
Adipose tissue is a connective tissue found throughout the body. It produces the hormone leptin (Greek “leptos” means “thin”) in response to food intake. Leptin binds to neuropeptide Y in the CNS neurons, producing a feeling of satiety after eating, thus affecting appetite and reducing the urge for further eating. Note that it has the opposite effect of ghrelin secretion from the stomach, but when leptin levels drop, the brain detects a state of starvation and the feeling of hunger increases. These two hormones are the subject of much research related to obesity.
Skin
Skin produces cholecalciferol, which is an inactive hormone form of vitamin D3. It is formed when cholesterol molecules in the skin, in the form of 7-dehydrocholesterol, are exposed to ultraviolet radiation. Cholecalciferol then enters the bloodstream and is modified in the liver to form calcifediol. Calcifediol is then modified in the kidneys to form calcitriol, which is the active form of vitamin D3. Vitamin D plays an important role in bone formation.
Bone
Bones not only respond to hormones to maintain blood calcium homeostasis, but recent research shows they also have an endocrine function. Osteocytes have been found to produce two hormones (fibroblast growth factor 23 and osteocalcin) that act on kidney, pancreas and other body tissues influencing Vitamin D and glucose homeostasis.
Thymus
The thymus is an organ that is found behind the sternum and is most prominent in infants, becoming smaller in size through adulthood, replaced by adipose tissue that continues to produce angiogenic factors. The thymus is part of the immune system, with a role in maturation and immunocompetence of T-lymphocytes. The thymus also produces a group of hormones called thymosins, because they were first discovered from the thymus, but now are understood to be produced by many different tissues in the body. They appear to have an anti-inflammatory effect and stimulate tissue repair. Thymosin is also involved in neuroplasticity, and they may have clinical implications in the treatment of cardiovascular, infectious and autoimmune diseases as well as cancer.
| Organ | Associated Hormones | Main Effect |
|---|---|---|
| Heart | Atrial Natriuretic Peptide (ANP) | Reduces blood volume, pressure, and Na+ concentration |
| Gastrointestinal Tract | Gastrin, Secretin, and Cholecystokinin | Aid in the digestion of food |
| Kidneys | Renin | Stimulates production of angiotensin II |
| Calcitriol | Aids in the absorption of Ca2+ | |
| Erythropoietin | Triggers the formation of red blood cells in the bone marrow | |
| Adipose Tissue | Leptin | Promotes satiety signals in the brain |
| Skin | Cholecalciferol | Modified to form vitamin D |
| Thymus | Thymosins | Aid in the development of the immune system |

