9 Electrolytes and pH
Electrolytes
An electrolyte is any fluid that contains free ions. You have already learned about ions and ionic properties. Important ions in physiology include sodium, potassium, calcium, chloride and phosphate. These ions are used in maintaining protein structure and in cell communication, and generally can help maintain water balances throughout the body. We get electrolytes through ingestion. Drinks with “electrolytes” have salts (sodium and potassium) that help maintain ion levels for athletes that lose ions through sweat. Electrolytes are essential for life, but many people get too much (like too much sodium from salt in processed food), which can also disrupt proper physiological function.
pH
The hydrogen ion concentration (H+) of a solution is an important property, because biological systems contain functional groups whose properties are changed by changes in the hydrogen ion concentration.
Since the hydrogen ion concentrations are usually much less than one, and can vary over many orders of magnitude, a different scale is used to describe the hydrogen ion concentration—the pH scale. The pH is the negative logarithm (-log) of the proton concentration:pH = – log (H+). The log conversion reduces a tenfold change in hydrogen ion concentration to a one unit change in pH. The minus sign changes the negative numbers that would be obtained from log(H+) to positive ones. Since the pH scale is an inverse scale, the concentration of protons is high at low pH and low at high pH. A solution is said to be acidic if the pH is less than 7.0, and basic if the pH is more than 7.0. A solution is neutral if its pH is equal to 7.0.
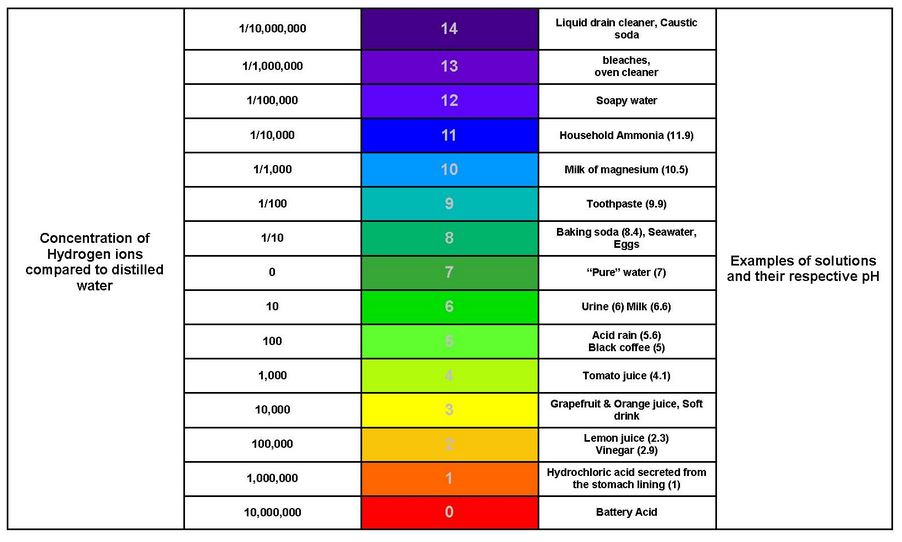
The image below shows the pH of a number of common fluids.
pH of various compounds[1]
Acid Dissociation and pH
For our studies, the Bronsted definition of an acid will be used. Here, we will define an acid as a proton donor and a base as a proton acceptor. Hydrochloric acid, like sodium chloride, is a strong electrolyte because it completely dissociates in aqueous solution into charged ions. Hydrochloric acid is also a strong acid, because when it completely dissociates it also completely donates all of its protons.
Many molecules are weak electrolytes and exist in an equilibrium (indicated by ⇌ in the general equation below) between the starting molecule and its dissociated parts. Thus dissociation can be seen as an acid (HA) in equilibrium with a proton (H+) and the corresponding conjugate base (A–).
In general: HA ⇌ A– + H+
Specifically for acetic acid: CH3COOH ⇌CH3COO– + H+
- Image: pH of Various Compounds Section: pH, Image description: Concentration of Hydrogen ions compared to distilled water, along with examples of solutions and their respective pH. pH 0 Concentration: 10,000,000. Example: battery acid pH 1 Concentration: 1,000,000. Examples: hydrochloric acid secreted from the stomach lining (1) pH 2 Concentration: 100,000. Examples: lemon juice (2.3), and vinegar (2.9) pH 3 Concentration: 10,000. Examples: grapefruit and orange juice, soft drinks pH 4 Concentration: 1,000. Example: tomato juice (4.1) pH 5 Concentration: 100. Examples: acid rain (5.6), black coffee (5) pH 6 Concentration: 10. Examples: urine (6), milk (6.6) pH 7 Concentration: 0. Example: “pure” water (7) pH 8 Concentration: 1/10. Examples: baking soda (8.4), seawater, eggs pH 9 Concentration: 1/100. Example: toothpaste (9.9) pH 10 Concentration: 1/1000. Example: milk of magnesium (10.5) pH 11 Concentration: 1/10,000. Example: household ammonia (11.9) pH 12 Concentration: 1/100,000. Example: soapy water pH 13 Concentration: 1/1,000,000. Examples: bleach, oven cleaner pH 14 Concentration: 1/10,000,000. Examples: liquid drain cleaner, caustic soda ↵

