7 Chemical Bonding and Molecules
Chemical bonds result when atoms of the same element (e.g., C-C) or different elements (e.g., C-O, C-N, O-H) combine into relatively strong, commonly neutral, structures. There are two major types of chemical bonds: ionic and covalent. Covalent bonds can further be divided into polar covalent and nonpolar covalent bonds. A polar covalent bond is a type of covalent bond that results in unique interaction between molecules. These polar bonds will interact with other polar bonds through an intermolecular attraction known as hydrogen bonding, such as that found between water molecules. Both the strong ionic and covalent chemical bonds and the weaker intermolecular forces are important in the functioning of the cell.
Ionic Bonds
Ions form when an atom or group of atoms gains or loses one or more electrons. When an atom gains electrons, it becomes a negatively charged ion, called an anion. When an atom loses electrons, it becomes a positively charged ion called a cation. Atoms with higher electronegativities tend to gain electrons and become anions, whereas those with lower electronegativities tend to lose electrons and become cations. The electrostatic attraction between a positively charged ion and a negatively charged ion is the basis of an ionic bond.
An ionic bond generally forms between an atom of low electronegativity and an atom of high electronegativity. In many cases this will be between a metal and a nonmetal. In this situation, one or more electrons is transferred from the atom with low electronegativity, which readily “gives away” its electrons, to the atom with high electronegativity, which strongly attracts those electrons.
Example: Sodium (Na) Atom Electrons to Chlorine (CL)
A sodium (Na) atom will transfer its one valence electron to a chlorine (Cl) atom, resulting in the formation of a sodium cation, Na+, and a chloride anion, Cl–. Because these are oppositely charged particles, they are attracted to each other and form table salt which is stable in air. When an ionic compound, like table salt, is put into water, it dissolves. This happens because the polar water molecule pulls these oppositely charged ions apart, as will be discussed further in the next module.
Covalent Bonds
After single elemental atoms, we can think of small molecules as the next level in chemistry’s hierarchy. Molecules result from the covalent bonding of two or more elements’ atoms.
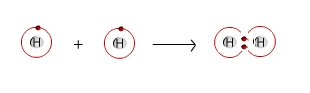
Covalent bonds are strong bonds in which electrons circling the atomic nucleus are shared. The nature of the covalent bond is determined by the number of electrons shared and the nature of the two elements sharing the bond. Two or more atoms held together by covalent bonds in a specified arrangement is called a molecule. The diagram below illustrates the covalent bond that forms between two hydrogen atoms to form a molecule of hydrogen. Nonpolar covalent bonds form between atoms of the same or similar electronegativities, most often two nonmetals.
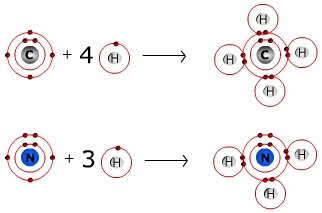
Each atom typically forms a specific number of covalent bonds when in a molecule with other atoms. The number of bonds that a particular atom will form is based on the atom’s valence electrons. Carbon for instance, which has four valence electrons, will form four bonds when it is in a molecule, as you can see from the diagram of methane below. Nitrogen, which has five valence electrons, will form three bonds, as seen in the ammonia molecule. The number of covalent bonds that a nonmetal will typically form is provided in this table for the biologically important elements.
| Atom | Mass | Valence Electrons | Covalent Bonds |
|---|---|---|---|
| H | 1 | 1 | 1 |
| C | 12 | 4 | 4 |
| N | 14 | 5 | 3 or 4 (e.g. NH4+) |
| O | 16 | 6 | 2 |
| P | 31 | 5 | 3 |
| S | 32 | 6 | 2 |
| Cl | 35 | 7 | 1 |
The number of bonds between two atoms, such as single bonds, double bonds or triple bonds, helps determine the stability of the atomic interactions. Double bonds, which share two pairs of valence electrons between two atoms, are very strong. The strong bond of carbon double bonded to oxygen is found in amino acids (these will be discussed later). The number of valence electrons shared also controls the “shape” of the atomic interactions. Carbon double bonded to oxygen forms a “flat” (planar) bond that does not rotate. This limits the shapes that the larger macromolecule, with repetitive double bonds, can form.
Formation of Hydrogen Molecule
Formation of Methane and Ammonia Molecules
Polar Covalent Bonds
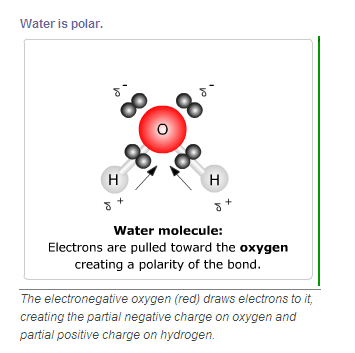
In a molecule such as hydrogen, the electrons are shared equally because each atom has the same electronegativity. However, in some molecules one atom is more electronegative than another, in which case the electrons are not shared equally. For example, in a water molecule, one oxygen atom is covalently bonded to two hydrogen atoms. Because an oxygen atom is more electronegative than a hydrogen atom, the oxygen atom draws the electrons being shared toward itself and away from the less electronegative hydrogen. When electrons in a covalent bond are shared in an unequal manner it is termed a polar, or polar covalent, bond. This unequal sharing of electrons results in the more electronegative element, in this example the oxygen atom, having a slightly negative charge and the less electronegative element, in this example the hydrogen atom, having a slightly positive charge. Molecules with polar bonds have characteristics of both ionic and covalent bonds. Whether or not a molecule is polar has significant implications on how that molecule interacts with other molecules and ions in biological systems.
Water and Hydrogen Bonding
Water is the basis of life. Without it, life is not possible. Water accounts for up to 75 percent of the weight of the human body. Water provides a relatively stable medium in which chemical reactions can take place. Water’s unique chemical properties (as a solvent with a high boiling point and high heat capacity) make it essential for homeostasis. The body’s thermoregulation relies on water’s high heat capacity to buffer it against swings in external temperature. Cooling of the body is carried out by evaporative water loss—perspiration. Water is also vital as a transport medium. Oxygen is carried by red blood cells suspended in serum, which is mostly water. Nutritional substances are dissolved in water and transported to cells. Water is used to dissolve and dilute waste molecules. If the body is deprived of water for very long, death will result.
Water has several properties that contribute to its suitability to support life as we know it. One of these properties is that water is a polar molecule. Oxygen is more electronegative than hydrogen and draws the electrons that it shares in the covalent bond towards itself. Because water is polar, the partial positive end of one water molecule will be attracted to the partial negative end of a neighboring water molecule. This attraction is called a hydrogen bond. Hydrogen bonding occurs between partially negatively charged atoms with high electronegativity—oxygen, nitrogen or fluorine—and partially positively charged hydrogen atoms that are bonded to oxygen, nitrogen or fluorine atoms.
Hydrogen bonding, which is referred to as an intermolecular attraction, is a critical interaction within the cell. It is the principal force that holds the tertiary structure of proteins, carbohydrates and nucleic acids together and the overall stability of these molecules is due in part to the cumulative effect of the large number of hydrogen bonds found in the functional structures. Hydrogen bonds are found in and between a variety of molecules. For example, the enormous number of hydrogen bonds between strands of plant cellulose provide the strength and structure of the plant cell wall. As another example, wool (sheep hair) has lots of proteins with an enormous number of hydrogen bonds that provide the curly structure of individual wool fibers. These curly fibers trap air spaces which makes wool such a good insulator. When washed at high temperatures, these hydrogen bonds are broken and the wool fibers will lose their shape, probably damaging any wool clothing.
A water molecule attracts four other water molecules towards itself. One molecule to each of the two free pairs of electrons in the oxygen atom valence shell, and one to each of the hydrogen atoms covalently bonded to the oxygen. As the water molecules associate with each other they have a defined structure dictated by the tetrahedral geometry of the electrons around the oxygen atom as seen in the figure below. The partially positive hydrogen atoms are attracted to the free electron pairs from other water molecules while the partially negative charge on the oxygen’s free electron pairs are attracted to the partially positive hydrogen atoms from another water molecule.
The polar character of water that results in its ability to form extensive hydrogen bonding networks gives rise to several properties of water that are important to sustaining life. The following is a list of a few of these properties.
- Water has a high surface tension. This allows it to enter small structures (capillary action), allowing water to be transported in plants.
- Ice is less dense than water. If ice were more dense, lakes would freeze from the bottom up, never completely thawing in the summer.
- High heat is required for vaporization of water (a large amount of heat is required to convert liquid water to a gas). The high heat required for vaporization of water allows an organism to cool via evaporation of sweat. It also prevents the body from losing too much water due to evaporation (sweating) when you run a fever or are in a hot climate.
- Water has a high specific heat capacity (a large amount of heat is required to raise the temperature of one gram of water one degree Celsius). This means that heat produced by biochemical reactions within an organism doesn’t raise the temperature of the organism.
- Water has a high dielectric constant (a measure of the polarity of the polar covalent bond). This reduces ionic attraction, allowing salts to dissolve.

