39 Nervous System Levels of Organization
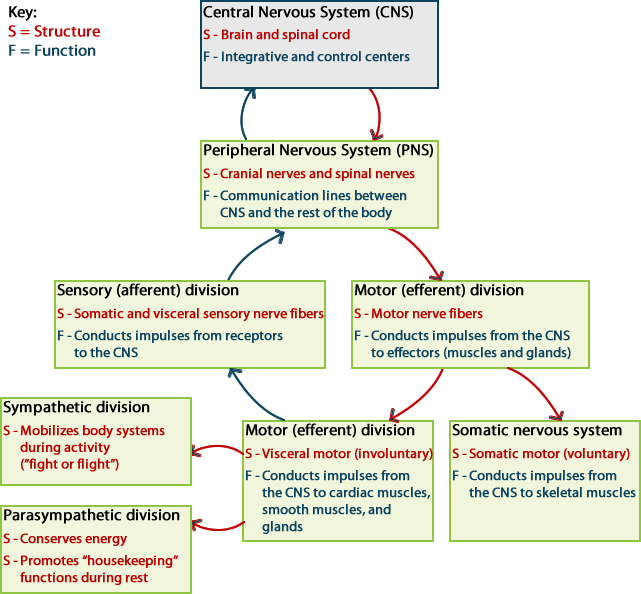
The nervous system consists of two major divisions:
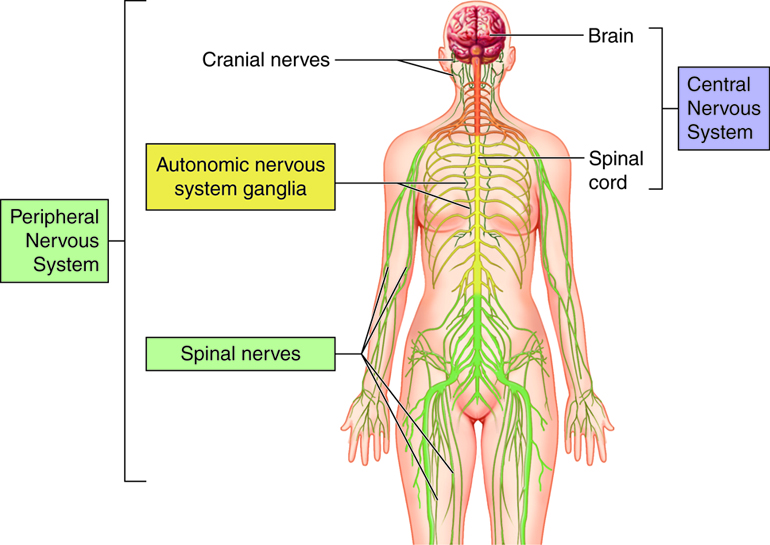
- The central nervous system (CNS) consists of the brain and the spinal cord, which are enclosed in the skull and vertebral column, respectively.
- The peripheral nervous system (PNS) consists of all the neural tissue outside of the brain and spinal cord. The PNS includes the cranial nerves and spinal nerves, sensory receptors and ganglia (cell bodies (somas) of neurons that lie outside the CNS). The nerves connect all other parts of the body with the CNS.
The peripheral nervous system has several subdivisions. It is first divided based on function into sensory (afferent) and motor (efferent) divisions. Each of these is further subdivided into somatic and autonomic (visceral) divisions.
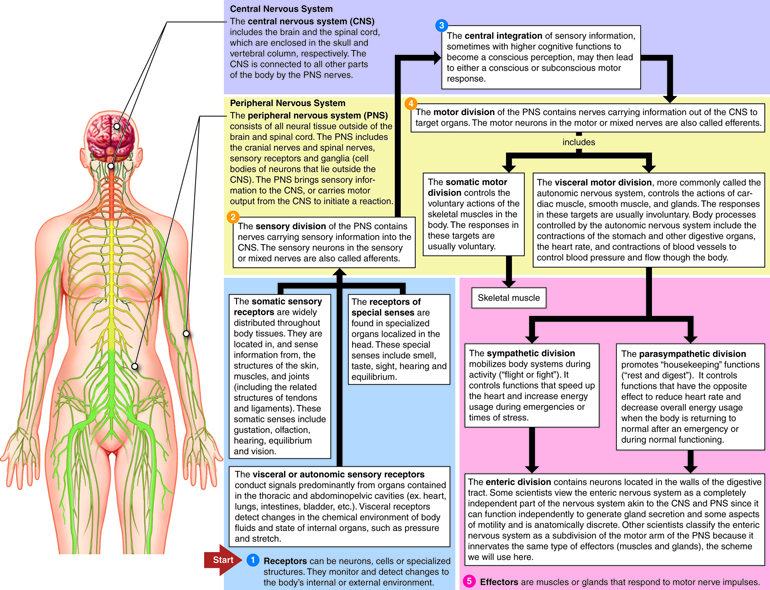
The nerves that comprise the peripheral nervous system can be divided into two divisions based on whether information is traveling into the CNS or information is leaving the CNS. The sensory division of the PNS contains nerves carrying sensory information into the CNS. These sensory nerves are also called afferents (carrying toward). The motor division contains nerves carrying information out of the CNS to target organs. These motor nerves are also called efferents (carrying away).
The sensory (afferent) division of the PNS has two subdivisions. The somatic sensory division conducts signals from receptors located in the skeletal muscles and skin. The visceral or autonomic sensory division conducts signals predominantly from organs contained in the thoracic and abdominopelvic cavities (ex. heart, lungs, intestines, bladder, etc.).
The motor (efferent) division of the PNS is also subdivided into somatic and visceral divisions. The somatic motor division controls the voluntary actions of the skeletal muscles in the body. The visceral motor division, more commonly called the autonomic nervous system, controls the actions of cardiac muscle, smooth muscle, and glands. The responses in these targets are usually involuntary.
The autonomic nervous system (ANS) is further subdivided into the sympathetic division and the parasympathetic division. Generally, the sympathetic division is involved in getting the body ready to respond to a physical challenge or an emotional threat, classified historically as the “fight or flight” division of the ANS. The parasympathetic division functions in opposition to the sympathetic nervous system. It is responsible for “rest and digest” activities, and is involved in salivation, digestion, urination and defecation.
Neurons
Neurons are considered the simplest functional unit of nervous tissue. They are long-lived (most live for your entire life), electrically active cells that consume a lot of energy. Neurons are capable of responding to stimulation, conducting electrical signals, and secreting chemicals that allow them to communicate with other cells. They cannot usually regenerate if damaged since most neurons do not retain the ability to divide. Neurons have anatomically and functionally distinct regions for receiving, integrating and sending information from one part of the body to another.
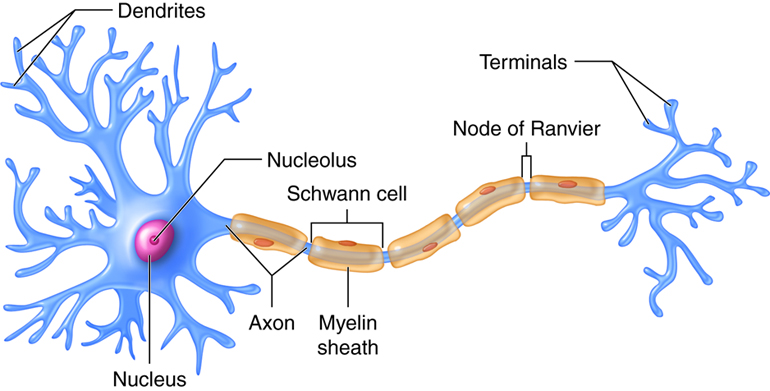
A typical neuron, like that shown above, has two distinct processes or cytoplasmic extensions on either side of a soma (cell body). On one side of the soma are short, tapering processes called dendrites (Greek, dendreon – tree). Most neurons have many, highly branched dendrites, although they may have as few as one. Dendrites receive information from other neurons and transfer it to the cell body. The greater the number of dendrites, the more information the neuron can collect to use during decision making.
The soma (cell body) is the region of the neuron that integrates all the incoming information from the dendrites. The cell body is somewhat spherical in shape and for humans, typically ranges in size from 5 – 100 microns in diameter. The soma contains the neuron’s nucleus and housekeeping organelles (e.g. mitochondria, lysosomes, Golgi complex, rough endoplasmic reticulum, etc.). The soma is the only site in a neuron that can synthesize proteins, neurotransmitters, or materials needed for cell maintenance and repair. The soma is similar to the dendrites in that it can also receive inputs from other neurons. The incoming information is coded as electrical signals that are integrated to determine what, if any, response is required. If the incoming signal is large enough, it travels to the process on the other side of the soma called the axon. If the incoming signal is small, it dies out before it reaches the axon.
The axon functions like a cable, relaying electrical signals away from the cell body towards other neurons or cells (e.g. muscles, glands). Axons are also called nerve fibers. The axon has three regions. As it emerges from the cell body, the axon forms a structure called the axon hillock, a tapered region that contains the initial segment, or trigger zone, where propagating electrical signals called action potentials are initiated or generated. The next part of the axon is the longest, typically a single, thin (.5 – 3 microns), almost constant diameter process that extends to a target. Axons can be long, short or in between.
The axon may travel to its target as a single fiber, but some axons form branches called collaterals, so that they can interact with not just one, but many target cells. The third region of the axon is found when it reaches its target. Here the axon branches extensively forming the synaptic terminals (terminal arborization). Each branch ends with a small swelling called a synaptic knob, which contains vesicles filled with chemical messengers (neurotransmitters) that conduct the signal to the next cell.
Neuron Anatomy
Neurons are classified according to 2 different characteristics: their morphology (anatomical or structural features) and their function. Although we describe both here, the functional classification will be used predominantly in later units.
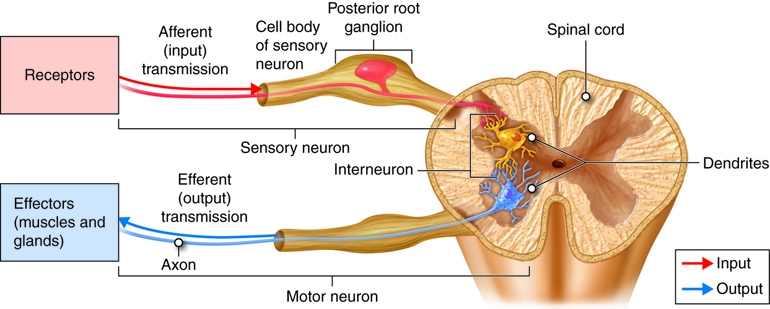
Functional classification of neurons is based on the direction of information flow along axons relative to the CNS. Based on this criterion, there are 3 types of neurons: sensory neurons, interneurons, and motor neurons.
Sensory (afferent) neurons are specialized for detection of sensory information (e.g. light, pressure, vibration, temperature, chemicals, etc.). They transduce physical and chemical stimuli into electrical signals and transfer this information from the periphery towards the central nervous system for processing. In many cases, sensory neurons have their dendrites, soma and a part of their axon residing outside the CNS with axon terminals forming connections (synapses) with other neurons within the CNS.
Interneurons (association neurons) are located entirely within the central nervous system (with the dendrites, soma and axons of the cell all residing within the CNS). Interneurons are also referred to as association neurons, in part because they are sandwiched between sensory and motor neurons where they integrate and distribute sensory information and coordinate motor output. Interneurons account for 90% of all neurons of the CNS and therefore are the most numerous neurons in the body. Almost all interneurons are multipolar (see below).
Motor (efferent) neurons carry impulses or motor commands away from the central nervous system to effectors/target organs (e.g. muscles and glands). Most motor neurons have dendrites and cell bodies in the CNS and axons that exit the CNS to form peripheral nerves that travel to effectors (targets).
The structural classification of neurons is based on the number of processes that extend from the soma. There are 4 basic neuronal structures like those shown in the figure below though there are many subtle variations on each theme.
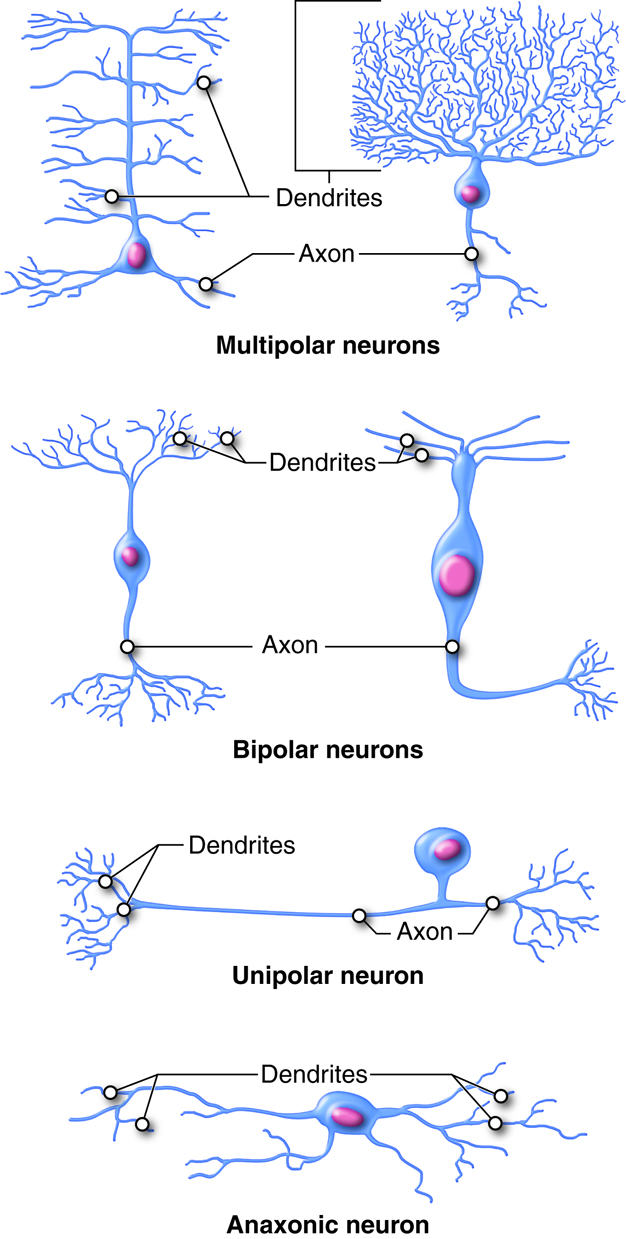
Bipolar neurons have a single dendrite extending from one side of the cell body and a single axon extending from the other side. Bipolar neurons are small cells, typically extending for less than 30 microns from dendrite to axon terminal. There are not many true bipolar cells in the body. A few examples are found in the special sense organs for vision and olfaction (smell).
Unipolar or pseudounipolar neurons have a single process that emanates from the cell body. The single process has dendrites on one end and the rest of the process is an axon. Most sensory neurons of the peripheral nervous system are unipolar neurons. The dendrites are located in the periphery, where stimuli are detected. The sensory information travels on the dendrite toward the soma (usually located ganglia just outside the CNS).. The axon stretches into the CNS at the spinal cord.
Multipolar neurons have two or more dendrites on one side and a single axon on the other side of the soma. Multipolar neurons are the most common neurons in the CNS. One example are motor neurons which have dendrites and somas located in the spinal cord and axons that leave the CNS to innervate skeletal muscles.
Anaxonic neurons are small, stellate (star-shaped) cells with processes that all look alike with no apparent axon. Anaxonic neurons can be found in the central nervous system, the retina, and in the adrenal medulla. Their functions are not well understood.
Glial Cells
Most neurons are surrounded by glial cells (neuroglia), the other cell type found in the nervous tissue. Glial cells are the supportive cells of the nervous system and are 10 times more numerous than neurons. The most well defined role for neuroglia is to provide structure to the delicate nervous tissue. They fill the space between neurons, serving as mortar or “glue” and thus hold nervous tissue together. Unlike neurons, glial cells retain the ability to divide throughout one’s lifetime. When neurons are injured, neuroglia are stimulated to divide and form glial scars. Glial cells have different shapes and sizes and their processes are indistinguishable in contrast to the distinct axon and dendrites found in neurons.
There are 6 types of glial cells, 4 types are found in the CNS and 2 types in the PNS. The CNS neuroglia are: astrocytes; oligodendrocytes; microglia, and ependymal cells. The 2 types of glia found only in the peripheral nervous system (PNS) are satellite cells and Schwann cells.
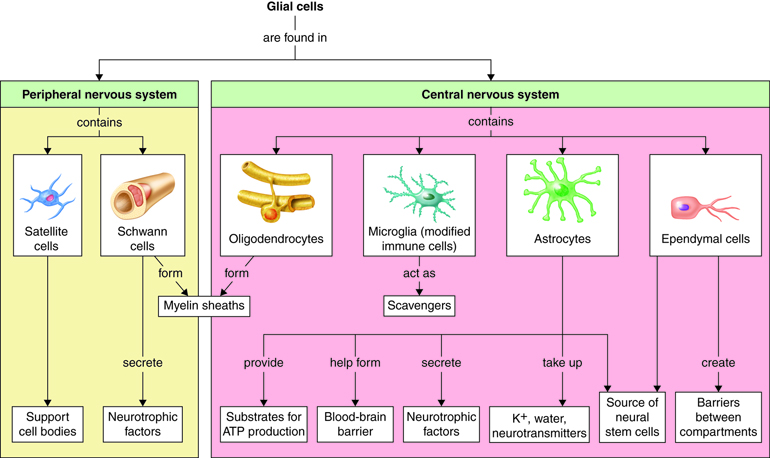
CNS Glial Cells
Astrocytes are star-shaped neuroglia and are the most numerous cells in the central nervous system. They make up half of all cells in the brain. Astrocytes provide a structurally supportive framework for neurons with their processes wrapping most non-synaptic regions of neurons in gray matter and covering the entire outer surface of the brain to form the glial – pia (connective tissue meninx) interface. Astrocytes help form the protective blood-brain barrier by encircling CNS capillary endothelial cells and stimulating the cells to form tight-junctions. They help to maintain the concentration of chemicals in the extracellular space and remove excess signaling molecules. Astrocytes also react to neural tissue damage by forming scar tissue in the damaged space.
Oligodendrocytes are glial cells of the CNS that wrap and insulate axons and give the CNS white matter its characteristic glossy, white appearance. Oligodendrocytes have a large soma with up to 15 processes. The processes reach out to axons of nearby neurons and wrap around them (like wrapping tape around a pencil) forming a high resistance sheath called myelin. Myelin insulates a small region of the axon (prevents ions from leaking out into the extracellular fluid), which facilitates signal propagation down the axon towards the synaptic terminal. A single oligodendrocyte’s processes will wrap axons of numerous different neurons. Processes from many different oligodendrocytes contribute to the myelin sheath of a single neuron’s axon.
Microglia are small highly mobile, phagocytic neuroglia that protect nervous tissue from pathogen infection, remove debris and waste, and may play a role in remodeling of the synapse that occurs during development and with learning. About 10-15% of CNS glial cells are microglia. Microglia are derived from monocytes and thus are more closely related to white blood cells than to the other glial cells. Since cells of the immune system cannot penetrate the blood brain barrier, microglia serve as brain macrophages, destroying foreign invaders, promoting inflammation and destroying cancer cells and cells infected with virus. Clusters of microglia in nervous tissue provide pathologists with evidence of recent injury.
Ependymal cells are cuboidal-shaped glial cells that are joined together to form a continuous sheet lining the fluid-filled ventricles and central canal of the brain and spinal cord. Ependymal cells produce and secrete cerebrospinal fluid (CSF), the fluid that bathes the tissues of the CNS. The basal side of the cell has rootlets that anchor the cells to the underlying tissue. The apical surface is marked by cilia, which helps circulate the CSF.
PNS Glial Cells
The remaining two glial cells, Schwann cells and satellite cells, are found solely in the peripheral nervous system.
Schwann cells are analogous in function to oligodendrocytes (found in the CNS). They insulate the axons of peripheral nerves in one of two ways. A Schwann cell can wind its way round and round the axon (up to 100 times), while squeezing its cytoplasm out of the way (much like a toothpaste tube could be wrapped around a pencil), forming a myelin sheath. Like myelinating a single fiber in the CNS, which requires many oligodendrocytes, a complete myelin sheath in the PNS requires many Schwann cells. Schwann cells can also envelop PNS axons without forming a myelin sheath. Instead of wrapping a single axon many times, the Schwann cell forms an envelope around a bundle of unmyelinated axons.
Additionally, Schwann cells can also assist in the regeneration of a damaged peripheral nerve. If a peripheral nerve is damaged, it may regenerate if its soma is undamaged and the neurilemma (the plasma membrane of the Schwann cell) enveloping it is intact.
Satellite cells are found surrounding neural somas in peripheral ganglia (collections of cell bodies located outside the CNS). Satellite cells resemble CNS astrocytes and are thought to have similar functions, providing structural support and regulating the chemical environment.
Axons: White and Gray Matter
Neuronal axons in the CNS and PNS can be devoid of a glial cell wrap (unmyelinated) or they can be discontinuously wrapped by glial cells along their entire length (myelinated). In a myelinated axon, the bare regions where the sheath is interrupted are called Nodes of Ranvier. The myelinated segments between consecutive nodes of Ranvier are called internodes. The myelin sheath changes the appearance of axons as well as their electrical properties. Myelinated axons appear white when viewed by the naked eye in contrast to areas where neuronal cell bodies are concentrated which appear gray.
Neurophysiology
Neurons produce electrical signals as a way of conveying information from one place in the body to another place very quickly, at speeds up to 100 meters/second (200 miles per hour). These rapidly traveling electrical signals allow you to perceive sensory stimuli, like the sound of a passing fire truck blasting its siren. Electrical signals, travelling in different neural pathways, coordinate motor responses that allow you to move your car out of the way of the fire truck, withdraw your hand from a dangerously hot pan, and rhythmically contract your diaphragm to breathe. Electrical signals arise as a result of movement of ions back and forth across the cell membrane of neurons. As ions move down their electrochemical gradients, they carry their charge with them, creating very miniscule but physiologically important electrical currents. These ionic currents flowing across membranes are the basis for the propagating electrical signals that underlie all nervous system functions. In this section, we’ll explore how neurons generate these electrical signals.
Cell Membrane Voltage
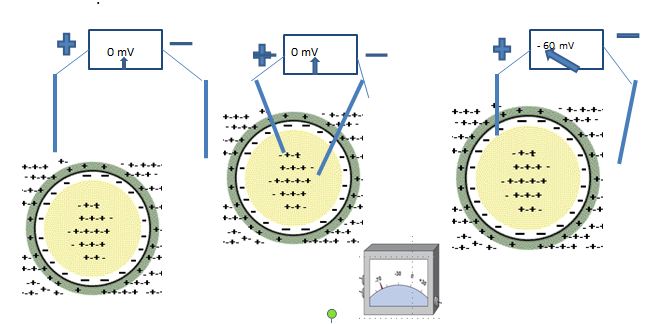
All living, eukaryotic cells have a transmembrane potential (a difference in charges between the intracellular and extracellular fluid). While the cell is at rest (i.e., unstimulated), the transmembrane potential is stable and is called the resting membrane potential (RMP). Right at the cell membrane, there is a little excess negative charge on the inside of the cell membrane and a little excess of positive charge on the outside. Because separation of charges creates a voltage, a very small probe on a voltmeter can be used to measure the voltage across the cell membrane. By convention the voltage outside the cell is set to zero. In a typical cell, the voltage recorded across the membrane is between -60 and -90 millivolts(-.06 to -.09 volts) with the negative sign indicating that the inside of the cell is negative with respect to the outside. Some cells have the ability to transiently alter their transmembrane potential (excitable cells), while others do not (non-excitable cells).
Non-excitable cells (ex: intestinal epithelial cells) have a stable and unchanging RMP. Excitable cells, like neurons and muscle, have a membrane potential that can fluctuate under certain conditions, with each fluctuation representing a signal produced by the cell. These fluctuations may be small and local to a region of a cell membrane (often called local or graded potentials) or larger in magnitude and travel along the length of the cell. These latter potentials, called action potentials, always lead to some response by the cells. In a neuron, action potentials lead to neurotransmitter release.
Ion Concentrations
To understand the basis of the resting membrane potential, we must first investigate the composition of body fluids – intracellular fluid (ICF) and extracellular fluid (ECF). Recall that ICF and ECF are composed of salts like NaCl and KCl that dissociate into their ionic components when placed in aqueous solutions. These ions are the mobile charges in body fluids that move between compartments to generate electrical currents along cell membranes.
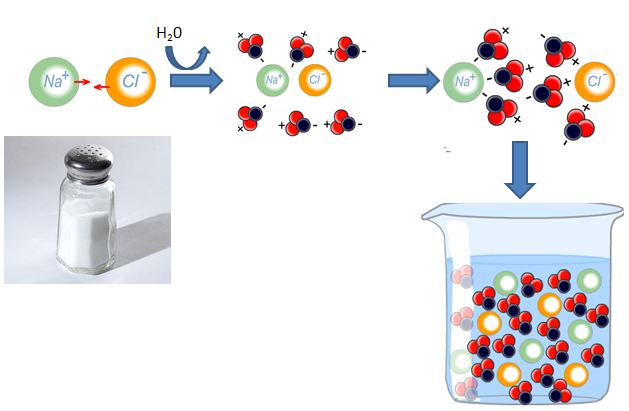
The ECF and ICF have high concentrations of different salts, which leads to differing concentrations of individual ions in the ECF versus the ICF. Recall from your earlier studies that sodium (Na+), chloride (Cl–), and calcium (Ca2+) are more highly concentrated in the ECF, while potassium (K+) and large anions (An–) are more highly concentrated in the ICF.
| Ion | Extracellular Fluid (mM) | Intracellular Fluid (mM) |
|---|---|---|
| K+ | 5 | 150 |
| Na+ | 145 | 15 |
| Cl- | 108 | 10 |
| Ca2+ | 1 | 0.0001
|
This table does not include all the ions, but only those that are major contributors to the membrane potential and changes in the membrane potential.
Neuron Resting Membrane Potential
The resting membrane potential arises due to the combined effects of three factors, which determine what ions move across the cell membrane in an unstimulated cell (at rest).
Ions are not evenly distributed between the inside and the outside of a cell. As we just learned, sodium is nearly 10 times more concentrated outside the cell than inside. Conversely, potassium is nearly 30 times more concentrated inside the cell than outside. The uneven distribution of ions leads to concentration gradients across the cell membrane. Given the opportunity, ions will move down their concentration gradient (i.e., from an area where they are highly concentrated to an area where they are less concentrated). So, given the chance, sodium ions would move into the cell and potassium ions would move out of the cell based on their respective concentration gradients.
The ionic composition of body fluids is tightly regulated. Small increases or decreases in ion concentrations can disrupt normal functions of the brain, heart, skeletal muscle or other organs. In many instances, you or one of your loved ones may need to receive treatment for an electrolyte imbalance. These situations might be as simple as that restoring blood lost in an accident, or receiving fluids to rehydrate you following a soccer tournament.
However, the cell membrane is not freely permeable to ions. Ions cannot freely cross the plasma membrane because of its structure. The lipid core of the cell membrane is hydrophobic and does not allow charged molecules to pass through it. Rather the cell membrane is selectively permeable, meaning it allows certain ions to pass. You know that the ions do not pass directly through the cell membrane, but rather pass through ion channels. The membrane is permeable to a specific ion if there are open channels for that ion. Recall that ion channels open and close based on the presence of electrical or chemical stimuli. Voltage-gated channels open at specific membrane potentials and are either inactivated (while the stimulus persists) or close when the membrane potential changes. Ligand-gated channels open when they bind chemicals and close when the chemical is no longer bound.
At rest, the cell membrane is most permeable to potassium because there are more open potassium channels at the resting membrane potential than channels for any other ion. As a result, potassium “leaks” out of the resting cell. The resting membrane is less permeable to sodium, and, at rest, a small amount of sodium “leaks” into the cell.
If these were the only things happening in the resting cell, the resting membrane potential would not be stable, but rather the net movement of potassium ions would cause the membrane potential to change. Ions move not only based on their individual concentration gradients, but they also move based on charge attraction and repulsion. Ions move away from like charges (ex. sodium and potassium ions move away from each other) and move towards opposite charges (ex. potassium ions would move toward chloride ions). The net movement of a particular ion is influenced by its electrochemical gradient (the balance of its concentration gradient and any charge attraction or repulsion).
One final factor also plays a role in determining the RMP. The sodium potassium pump operates continually in living cells. At maximum capacity, it pumps 3 sodium ions out of the cell and 2 potassium ions into the cell, and hydrolyzes 1 ATP to provide the energy for the ion transport.
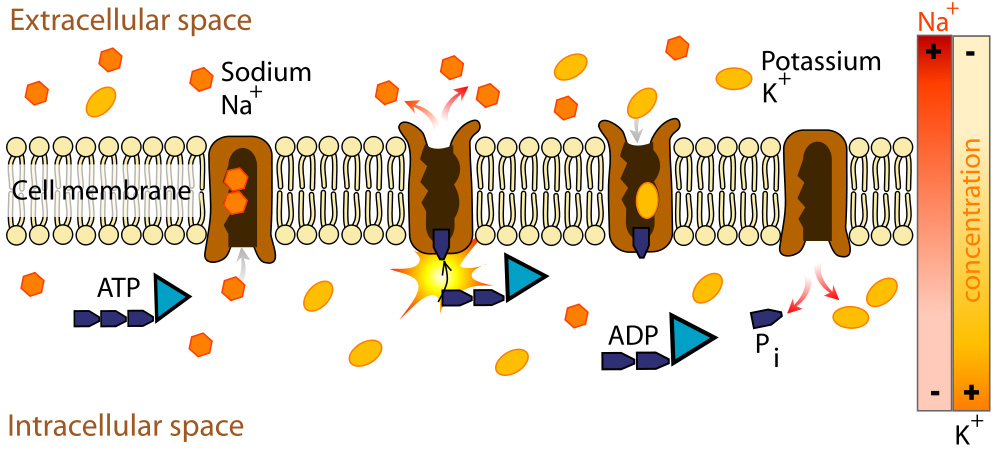
The sodium potassium pump is electrogenic (there are an uneven number of charges transported into and out of the cell resulting in a net charge associated with each exchange cycle). Since 3 sodium ions leave the cell and only 2 potassium ions enter the cell, there is a net negative charge on the inside of the cell due to the sodium potassium pump.
Neuron Electrical Response
Neurons can be excited by various stimuli such as light, chemicals, heat or pressure. These stimuli, which are typically received at the dendrites, cause small, localized changes in membrane voltage. The stimuli open ion channels in the membrane, which allows specific ions to flow in or out of the cell. This ion movement produces a change in the membrane voltage around the area of the open channels. These local shifts in membrane potential are called local (or graded) potentials. Local potentials have the following characteristics:
They are graded, which means the change in membrane voltage that occurs is proportional to the size of the stimulus. A stronger stimulus can open more ion channels. A stimulus that lasts for a long time can either open more ion channels or keep channels open for a longer time. In either case, more ions are able to cross the cell membrane, which produces a larger change in membrane voltage.
They are decremental, meaning that the signal grows weaker as it moves farther from the site of stimulation. Ion channels are opened at the site of stimulation and that is where ions move across the cell membrane. As a result, there is a high concentration of ions right around the ion channels. Once the ions cross the membrane, they diffuse away from the channel and there are fewer and fewer ions as they move away from the open channels. Fewer ions results in a smaller change in membrane potential.
They are reversible. If the stimulus comes to an end, the ion channels close and resting membrane potential is re-established before the signal travels very far.
They can either excite the cell or inhibit the cell depending on what type of ion channel is opened. If the stimulus opens a sodium channel, sodium enters the cell and depolarizes (make the membrane potential less negative) the membrane around the open channels. If the stimulus opens a chloride channel, chloride ions enter the cell and make the local membrane potential more negative than the RMP (hyperpolarizes the cell). Depolarization excites the cell and makes it more likely to send a signal to other cells. Hyperpolarization inhibits the cell and makes it less likely to send a signal to other cells.
A stimulus can also affect potassium channels. If the stimulus causes potassium channels to open, the effect will be hyperpolarization of that area of cell membrane. Potassium leaves the cell through the open channels, which removes positive charges from the ICF making the inside of the cell more negative. If the stimulus closes potassium channels, the membrane will depolarize around the closed channels because fewer potassium ions are leaving the cell.
Neurons generally receive multiple stimuli at the same time – some may be excitatory and others inhibitory. The overall response of the neuron will depend on the net effect of all the stimuli. In some cases, the neuron will produce a signal that will travel to other cells. In other cases, no signal will be sent from the neuron.
Action Potentials
If there is adequate excitatory stimulation of a neuron, a signal called an action potential is generated. An action potential is a transient and marked shift in membrane potential that occurs when voltage-gated ion channels in the membrane open. A series of action potentials can rapidly carry information from the neural soma along the axon to the axon terminal. A sufficient number of voltage-gated channels must be present in the cell membrane to initiate an action potential. The dendrites and most of the soma lack enough voltage-gated ion channels for this. However, at the trigger zone, where the soma interfaces with the axon, there is a high concentration of voltage-gated channels. To create an action potential in a neuron, an excitatory local potential must reach the trigger zone and depolarize (a shift in membrane potential making it less negative or even positive) it to the threshold voltage needed to open the ion channels.
Two types of voltage-gated channel are responsible for the propagating action potentials in most neurons – a fast Na+ channel (a voltage-gated Na+ channel that opens quickly when stimulated) and a slow K+ channel (a voltage-gated K+ channel that opens slowly when stimulated) . Let’s take a closer look at the specific events of an action potential.
Excitatory local potentials reach the trigger zone and depolarize it. If the local potentials depolarize the membrane to threshold (the membrane voltage at which the voltage-gated channels are stimulated to open), these voltage-gated channels begin to open. The fast Na+ channel opens quickly, increasing the permeability of the membrane to Na+ that flows into the cell down its electrochemical gradient leading to further depolarization. This causes more fast Na+ channels to open, further depolarizing the membrane. As the membrane potential reaches 0 mV, the fast Na+ channels become “inactivated.” A second gate that works like a timer closes the channel. By the time all the fast Na+ channels are inactivated the membrane voltage has reached its peak.
As the fast Na+ channels are being inactivated, the slow K+ channels are finally opening. This increases the permeability of the membrane to K+. Potassium ions leave the cell moving down their electrochemical gradient, and the efflux of positive charge causes the membrane voltage to return toward the resting membrane potential (repolarization).
Slow K+ channels stay open longer than fast Na+ channels, so more K+ leaves the cell than Na+ entered. The removal of excess potassium ions causes the membrane potential to become more negative than the resting membrane potential. When this happens, we say the membrane is hyperpolarized.
The changes in membrane permeability and their relationship to the membrane potential can be seen in the following figure.
Action Potential Refractory Period
The duration of time that the membrane is hyperpolarized following an action potential is termed its refractory period. The refractory period is an interval of time during which that part of the membrane cannot be excited (to produce another action potential) or requires a larger than normal stimulus to be excited. The refractory period is divided into two parts based on whether or not the membrane can be stimulated to produce an action potential. During the absolute refractory period, the membrane cannot be stimulated to produce another action potential regardless of the strength of the stimulus. During the relative refractory period, the membrane can be stimulated to produce an action potential, but a stronger than normal stimulus is required.
The absolute refractory period lasts from the beginning of the action potential (when the membrane reaches the threshold voltage) until the fast Na+ channels reset to their resting state. As long as the Na+ channels are open or inactivated, a new action potential cannot be generated.
The relative refractory period continues from the end of the absolute refractory period until the membrane is no longer hyperpolarized (returns to the resting membrane potential). During hyperpolarization, slow K+ channels are still open, but are in the process of closing. In order to stimulate an action potential during this time, a very strong stimulus is needed to overcome the effect of potassium flowing out of the cell and depolarize the cell.
Action potentials within a particular cell are all identical regardless of stimulus strength. If the membrane at the trigger zone reaches the threshold voltage or a voltage above the threshold, a maximal action potential will be generated. If the threshold voltage is not attained, no action potential is generated (no signal is propagated). In this way, action potentials are all-or-none – a cell either fires a full action potential or no action potential at all.
The action potential at the axon terminal looks exactly like the action potential that was initially generated at the trigger zone. Since the signal does not change as it travels the length of the axon it is nondecremental. It should be noted that the action potential at the axon terminal is not the same one that originated at the trigger zone. Rather, a series of identical action potentials are generated as the signal travels toward the axon terminal.
If the membrane reaches threshold, an action potential will be initiated and the signal will be propagated down the entire axon. Once the events are set in motion there is no stopping them. The process is irreversible.
In contrast to local potentials, which can either excite or inhibit the membrane, action potentials are all excitatory (cause an initial depolarization of the membrane).
Synapses
Several neurons are generally needed to transmit a signal from one place in the body to another. So how does the signal pass from one neuron to the next along a neural pathway? The signal must be transmitted across the interface between successive neurons and we will learn how that is accomplished in this next section.
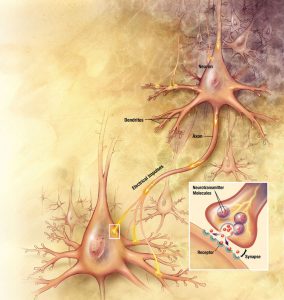
The term synapse means “coming together.” Where two structures or entities come together, they form a synapse. Although one can use the word synapse to mean any cellular junction, in physiology we traditionally limit its usage to: the junction of two neurons, the junction between a neuron and a target cell (for example, the neuromuscular junction), or the interface between adjacent cardiac muscle cells or adjacent smooth muscle cells. In the nervous system, a synapse is the structure that allows a neuron to pass an electrical or chemical signal to another cell.
Synapse Cells
The cell that delivers the signal to the synapse is the presynaptic cell. The cell that will receive the signal once it crosses the synapse is the postsynaptic cell. Since most neural pathways contain several neurons, a postsynaptic neuron at one synapse may become the presynaptic neuron for another cell downstream.
A presynaptic neuron can form one of three types of synapses with a postsynaptic neuron. The most common type of synapse is an axodendritic synapse, where the axon of the presynaptic neuron synapses with a dendrite of the postsynaptic neuron. If the presynpatic neuron synapses with the soma of the postsynaptic neuron it is called an axosomatic synapse, and if it synapses with the axon of the postsynaptic cell it is an axoaxonic synapse. Although our illustration shows a single synapse, neurons typically have many (even 10,000 or more) synapses.
Synapse Transmission
There are two types of synapses found in your body: electrical and chemical. Electrical synapses allow the direct passage of ions and signaling molecules from cell to cell. In contrast, chemical synapses do not pass the signal directly from the presynaptic cell to the postsynaptic cell. In a chemical synapse, an action potential in the presynaptic neuron leads to the release of a chemical messenger called a neurotransmitter. The neurotransmitter then diffuses across the synapse and binds to receptors on the postsynaptic cell. Binding of the neurotransmitter leads to the production of an electrical signal in the postsynaptic cell.
Why does the body have two types of synapses? Each type of synapse has functional advantages and disadvantages. An electrical synapse passes the signal very quickly, which allows groups of cells to act in unison. A chemical synapse takes much longer to transmit the signal from one cell to the next; however, chemical synapses allow neurons to integrate information from multiple presynaptic neurons, determining whether or not the postsynaptic cell will continue to propagate the signal. Neurons respond differently based on information transmitted by multiple chemical synapses. Let’s take a closer look at the structure and function of each type of synapse.
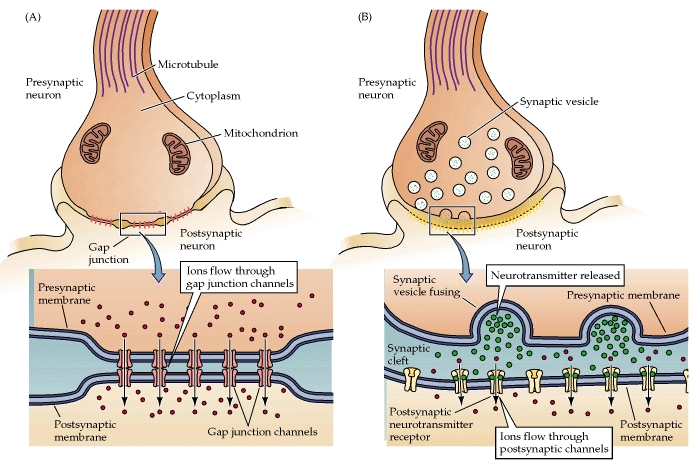
Electrical synapses transmit action potentials via the direct flow of electrical current at gap junctions. Gap junctions are formed when two adjacent cells have transmembrane pores that align. The membranes of the two cells are linked together and the aligned pores form a passage between the cells. Consequently, several types of molecules and ions are allowed to pass between the cells. Due to the direct flow of ions and molecules from one cell to another, electrical synapses allow bidirectional flow of information between cells. Gap junctions are crucial to the functioning of the cardiac myocytes and smooth muscles.
Chemical synapses comprise most of the synapses in your body. In a chemical synapse, a synaptic gap or cleft separates the pre- and the postsynaptic cells. An action potential propagated to the axon terminal results in the secretion of chemical messengers, called neurotransmitters, from the axon terminals. The neurotransmitter molecules diffuse across the synaptic cleft and bind to receptor proteins on the cell membrane of the postsynaptic cell. Binding of the neurotransmitter to the receptors on the postsynaptic cell leads to a transient change in the postsynaptic cell’s membrane potential.
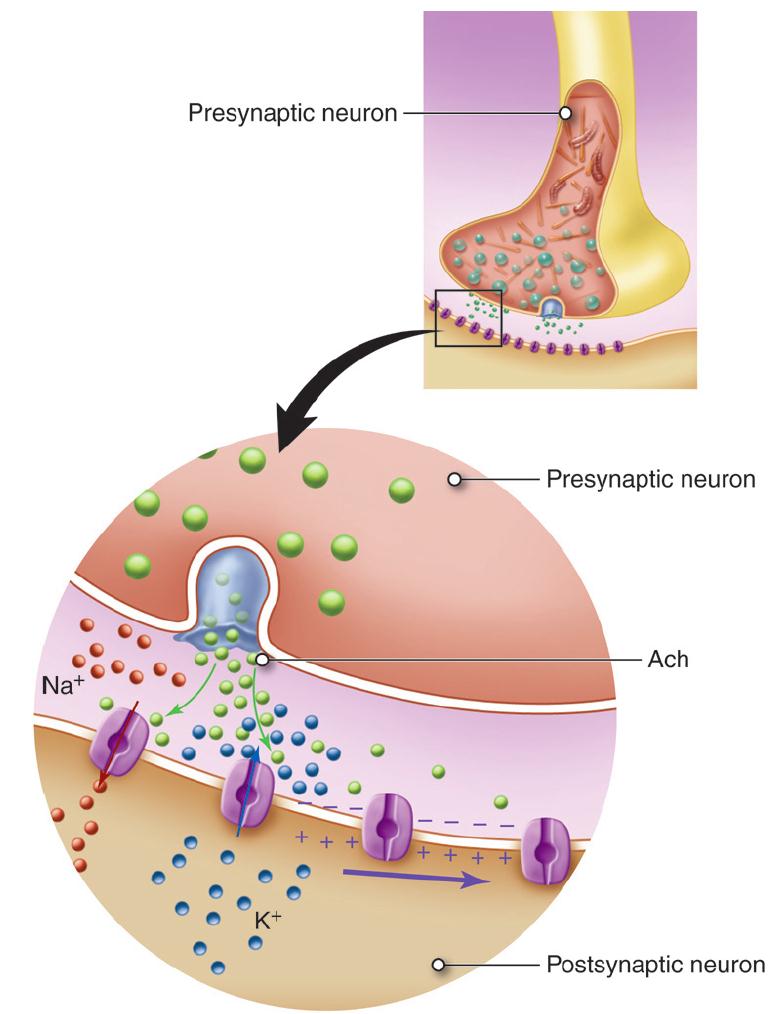
The process of synaptic transmission at a chemical synapse between two neurons follows these steps:
- An action potential, propagating along the axon of a presynaptic neuron, arrives at the axon terminal.
- The depolarization of the axolemma (the plasma membrane of the axon) at the axon terminal opens Ca2+ channels and Ca2+ diffuses into the axon terminal.
- Ca2+ bind with calmodulin, the ubiquitous intracellular calcium receptor, causing the synaptic vesicles to migrate to and fuse with the presynaptic membrane.
- The neurotransmitter is released into the synaptic cleft by the process of exocytosis.
- The neurotransmitter diffuses across the synaptic cleft and binds with receptors on the postsynaptic membrane.
- Binding of the neurotransmitters to the postsynaptic receptors causes a response in the postsynaptic cell.
The response can be of two kinds:
- A neurotransmitter may bind to a receptor that is associated with a specific ion-channel which, when opened, allows for diffusion of an ion through the channel. If Na+ channels are opened, Na+ rapidly diffuses into the postsynaptic cell and depolarizes the membrane towards the threshold for an action potential. If K+ channels are opened, K+ diffuses out of the cell, depressing the membrane polarity below its resting potential (hyperpolarization). If Cl– channels are opened, Cl– moves into the cell leading to hyperpolarization.
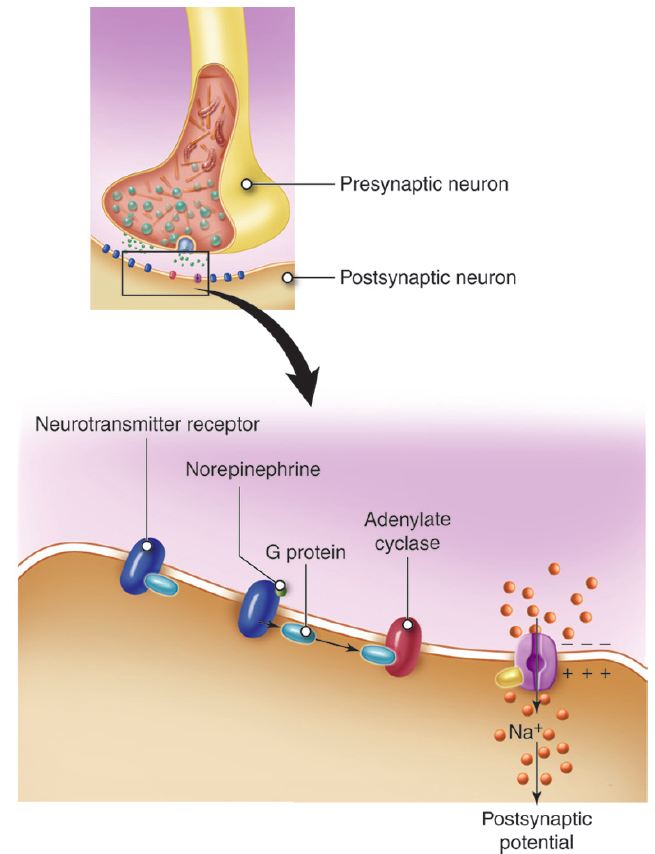
- The neurotransmitter may bind to a transmembrane receptor protein, causing it to activate a G-protein on the inside surface of the postsynaptic membrane. A cascade of events leads to the appearance of a second messenger (calcium ion, cyclic AMP (cAMP), or IP3) in the cell. Second messengers can have diverse effect on the cell ranging from opening an ion channel to changing cell metabolism to initiating transcription of new proteins.
Neurotransmitter Effects
The response of the postsynaptic cell to a neurotransmitter depends on the specific receptors that are present on its cell membrane. Most neurotransmitters can bind to more than one receptor found in the body and the cell’s response is dependent on which receptor is bound. Different receptors produce different cellular responses because they activate processes in the cell.
Example: Cholinergic Receptors
Let’s look at an example. Receptors that can bind the neurotransmitter acetylcholine (ACh) are termed cholinergic receptors – this is the type of receptor. There is more than one cholinergic receptor – the different cholinergic receptors are termed subtypes. One subtype, the nicotinic cholinergic receptor, opens a sodium channel when it binds ACh. Stimulation of a nicotinic cholinergic receptor leads to depolarization of the cell. Another subtype, the muscarinic cholinergic receptor, opens a potassium channel when it binds ACh. Stimulation of a muscarinic cholinergic receptor leads to cell hyperpolarization. Acetylcholine can either excite or inhibit the postsynaptic cell depending on whether that cell has the nicotinic or muscarinic receptor subtype.
In the example we just considered, both receptor subtypes were linked to distinct ion channels. It is also possible for one receptor subtype to be linked to an ion channel while another subtype leads to the production of a second messenger. In this case, the timing of the postsynaptic cell’s response is different. Opening an ion channel takes very little time compared to the complex signaling that occurs with a second messenger. The response is fast with a receptor linked to an ion channel and is slow with a receptor that leads to a second messenger cascade. Although slower, second messenger cascades can produce more diverse cellular effects and have the advantage of amplification. Binding of a single molecule of neurotransmitter can produce many molecules of the second messenger. In contrast, if the receptor opens an ion channel, a single molecule of neurotransmitter (or sometimes two molecules) is needed to open a single ion channel in the postsynaptic cell.
Neurotransmitter Classes
Neurotransmitters are organic molecules that allow neurons to communicate with each other and with target cells. Neurotransmitters fall into four classes based on their chemical makeup.
Acetylcholine (ACh) is a small molecule formed from acetate and choline. It is in a class by itself. Acetylcholine is the sole neurotransmitter used at the neuromuscular junction and is also the neurotransmitters used by the parasympathetic nervous system.
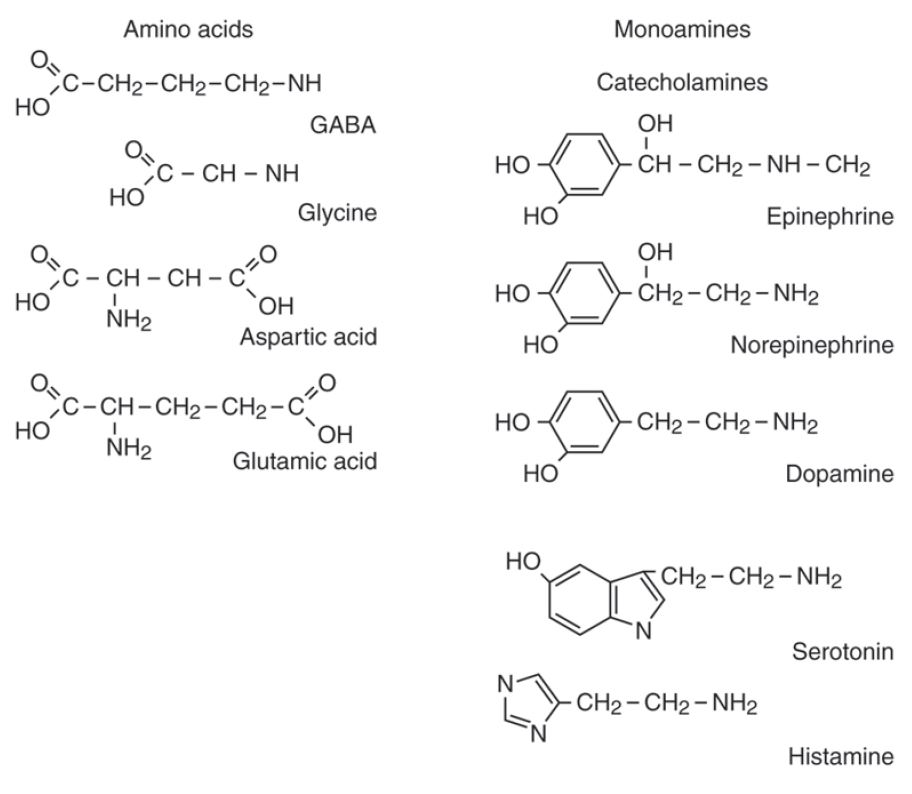
Some amino acids act as neurotransmitters. Glycine and γ-aminobutyric acid (GABA) are the most common inhibitory neurotransmitters in the spinal cord and brain, respectively. Glutamate (glutamic acid) and aspartate are excitatory neurotransmitters found in the brain and spinal cord, respectively.
Biogenic amines (monoamines) are formed from amino acids from which the carboxyl terminus is removed. Three of the biogenic amines, called the catecholamines, are grouped together as they are all derived from the same amino acid, L-tyrosine. The catecholamines include: norepinephrine (noradrenalin), epinephrine (adrenalin) and dopamine (dopamine can also be made from phenylalanine). Norepinephrine (NE) is the neurotransmitter of the sympathetic nervous system (your fight or flight response). Epinephrine (E) has similar effects to NE, but is less abundant. Dopamine is best known for its role in motor inhibition. Loss of dopamine producing neurons in Parkinson disease leads to dyskinesias (movement disorders). Catecholamines bind to adrenergic receptors. Other biogenic amines include serotonin and histamine.
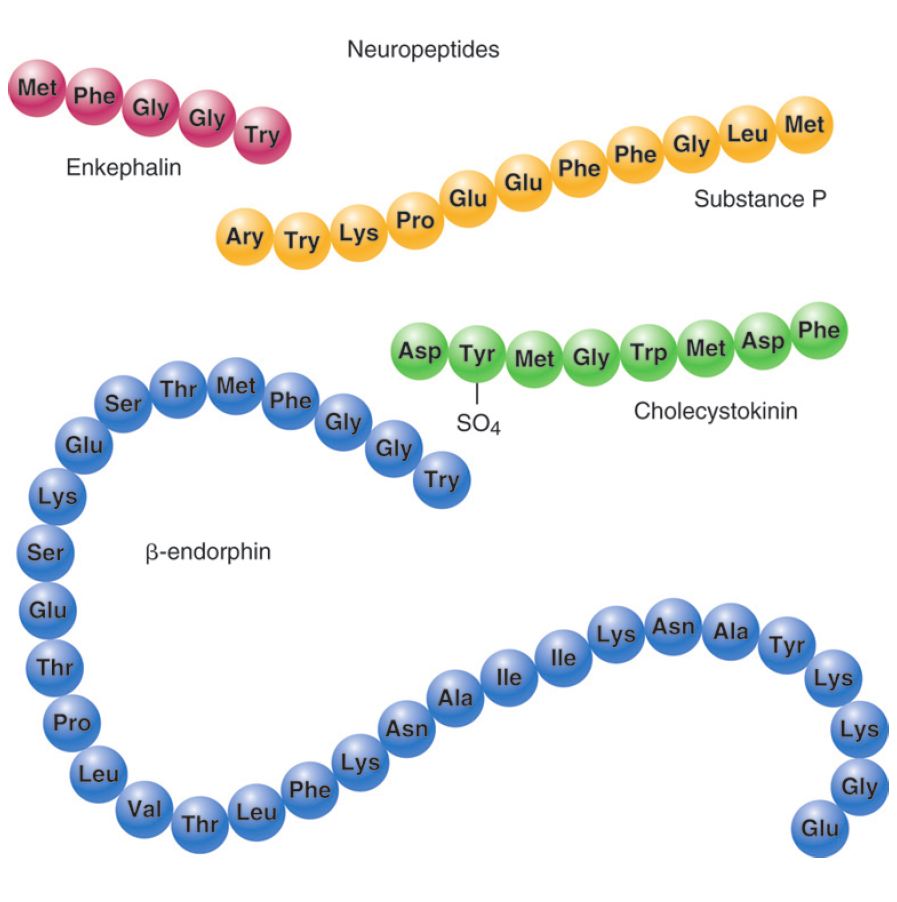
Neuropeptides are small proteins that function as neurotransmitters. They are the largest neurotransmitters and can range from just a couple of amino acids to as many as 40. An example of neuropeptide neurotransmitters is β-endorphin, the chemical associated with the elevated mood experienced with exercise.
The Autonomic Nervous System
Example: The Autonomic Nervous System at Work
Josie is sitting, having an outdoor lunch with friends when a large spider lands on her plate. She immediately freezes, the food in her mouth begins to feel like a wad of dry hay, and she nearly gags as she tries to swallow it. She feels her heart race and pound in her chest. After swallowing her food, it seems stuck in her throat and chest.
In this scenario, Josie had been sitting, relaxed and enjoying a meal. In this relaxed state, the body would have a heart rate that is at rest, active peristalisis (activity in muscles of the digestive system), ample activity in salivary glands and in digestive gland secretions, and bronchi that are not dilated. In this relaxed state, food can be easily processed due to ample amounts of saliva and digestive enzymes in the saliva released by the salivary glands into the mouth.. Salivation also facilitates swallowing by providing lubrication to the back of the throat and the esophagus. In this relaxed state, digestive fluids and enzymes in the intestines are actively produced and secreted so that food can be further processed and broken down (catabolized) for absorption of nutrients and glucose. While relaxed, Josie’s heart beats imperceptibly and her breathing is deep.
With the sudden appearance of the spider, the rate of Josie’s heart beat becomes more rapid, and it contracts more powerfully. In this vigilant state, Josie senses her rapid heart rate as well as the increased force of the contraction of her heart. She also senses a shift to rapid, shallow breathing that she tries to control. Her food seems lodged near the back of her throat as she struggles to swallow her food safely.
Within seconds of seeing the spider, Josie’s body systems shifted from reflecting calm to a state of panic and hyper-vigilance. These responses to the external threat prepare Josie to either fight or flee the situation. In the hyper-vigilant state, Josie’s pupils widen, she sweats and more blood is pumped through her blood vessels which permits more blood, chemicals, and hormones to flow to her skeletal muscles and respiratory system. As you can see, the effects on organ systems when in either of the states, relaxed or hyper-vigilant, are nearly opposite. These two states are controlled by two subsets of neural pathways that are part of the peripheral nervous system.
The peripheral nervous system itself has two main functional parts. These are the somatic nervous system, which controls voluntary movements of skeletal muscles, and the autonomic nervous system, which is the involuntary motor division of the nervous system.
The autonomic nervous system is further divided into the sympathetic nervous system and the parasympathetic nervous system. It is the complementarity of these two latter branches of the autonomic nervous system that drove the physiological changes in the “Spider Sat Down Beside Her” scenario above.
As evident from the impact of the sight of the spider on Josie’s ability to eat her meal, activity in the parasympathetic system is associated with a relaxing meal; on the other hand, activity in the sympathetic system is associated with alertness and vigilance. In keeping with the complementary functionality of the two systems, they have been given nick names. The parasympathetic branch works for “rest and repose” (also commonly known as “rest and digest”); the sympathetic branch is known for the “fight or flight” response (variously also known as “fight, flight or freeze;” “hyperarousal;” “acute stress”).
It will also be evident in later sections that both parasympathetic and sympathetic branches influence most organs, typically in opposition to each other. However, as will also be clear from the discussion going forward, physiologically this opposition is more complementary than antagonistic. One can give an example of a car, where the accelerator and brake are both necessary for its operation. The balancing that goes on between these two divisions of the ANS requires that most organs receive inputs from both. This is referred to as dual innervation. We will see examples of this later.
The Role of the ANS
The peripheral nervous system is divided into two functional subdivisions:
- Somatic Nervous System (SNS)
- Autonomic Nervous System (ANS)
The autonomic nervous system is further divided into the sympathetic and parasympathetic branches.
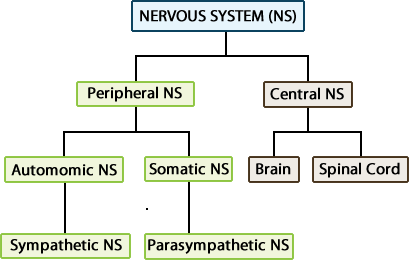
The somatic nervous system (SNS) deals with sensory input and voluntary motor (efferent) activities, while the autonomic nervous system (ANS) deals only with efferent (motor) signals from the CNS to control activities in the body that are distinct from those under conscious voluntary control. The targets of efferents are called effectors, and these are organs, muscles or glands. The autonomic nervous system is also called the visceral nervous system because it controls smooth muscle, cardiac muscle, and glands, which make up the viscera of the body.
Sensory information from internal organs (namely, the blood vessels, the heart, and the abdominopelvic organs) reaches the CNS levels of medulla, pons and hypothalamus, often without ever reaching sensory cortex of your cerebrum and, thereby, not reaching conscious awareness. These inputs elicit reflex responses through the efferent autonomic nerves. As necessary, the ANS neurons elicit appropriate reactions of the heart, the vascular system, and all the organs of the body in response to variations in the environment – physical or biochemical.
The following table compares basic structural and functional features of the SNS and the ANS:
| Somatic | Autonomic |
|---|---|
| Involves both afferent and efferent pathways | Involves only the efferent pathway |
| Voluntary activities, including locomotion (effectors are skeletal muscles) | Involuntary activities (effectors are cardiac muscle, smooth muscles, fat cells, and glands) |
| Efferent signals originate at the cerebral cortex as a conscious decision and activate neurons in the brainstem or spinal cord | Unconscious signals originate in hypothalamus, brain stem, and spinal cord and activate target neurons that lie in the peripheral nervous system |
| Brainstem and spinal cord neuron exerts direct control over skeletal muscle (one neuron system, where there are no intermediate synapses between the CNS and the target organs) | Target neurons in the peripheral nervous system are grouped in ganglia and their axons exert direct control over smooth muscle, cardiac muscle, glands, and fat cells (two neuron system, where the efferent neurons synapse once outside the CNS before the signals reach the target organs.) |
| Efferent axons are myelinated (fast conduction) | Postsynaptic axons are non-myelinated (slow conduction); presynaptic axons are myelinated |
| Neuromuscular junctions are specific and localized | Synapses at the target organs for the axons of the ANS may be diffuse (varicosities). |
| Target organs (skeletal muscles) are always stimulated into action | ANS used several different neurotransmitter molecules (predominantly ACh and norepinephrine, NE) |
Anatomy of Parasympathetic and Sympathetic Systems
The two parts of the autonomic nervous system are organized differently. The parasympathetic nervous system is derived from preganglionic neurons in the brainstem and from preganglionic neurons in the lateral horn of the spinal cord at sacral levels. Preganglionic neurons of the sympathetic nervous system lie in the lateral horn of the spinal cord at thoracic and lumbar levels of the spinal cord. This differential distribution of the preganglionic neurons of the two systems gives rise to the names “craniosacral division” for the parasympathetic nervous system and “thoracolumbar division” for the sympathetic division.
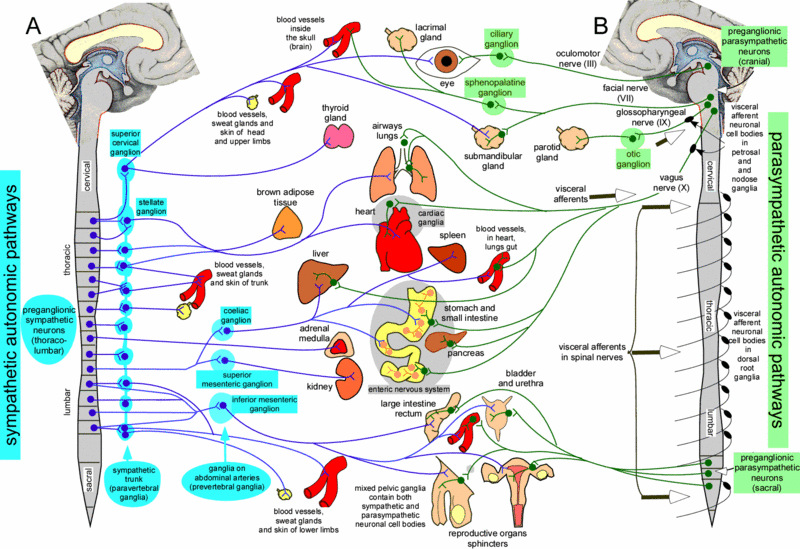
Effects of the ANS
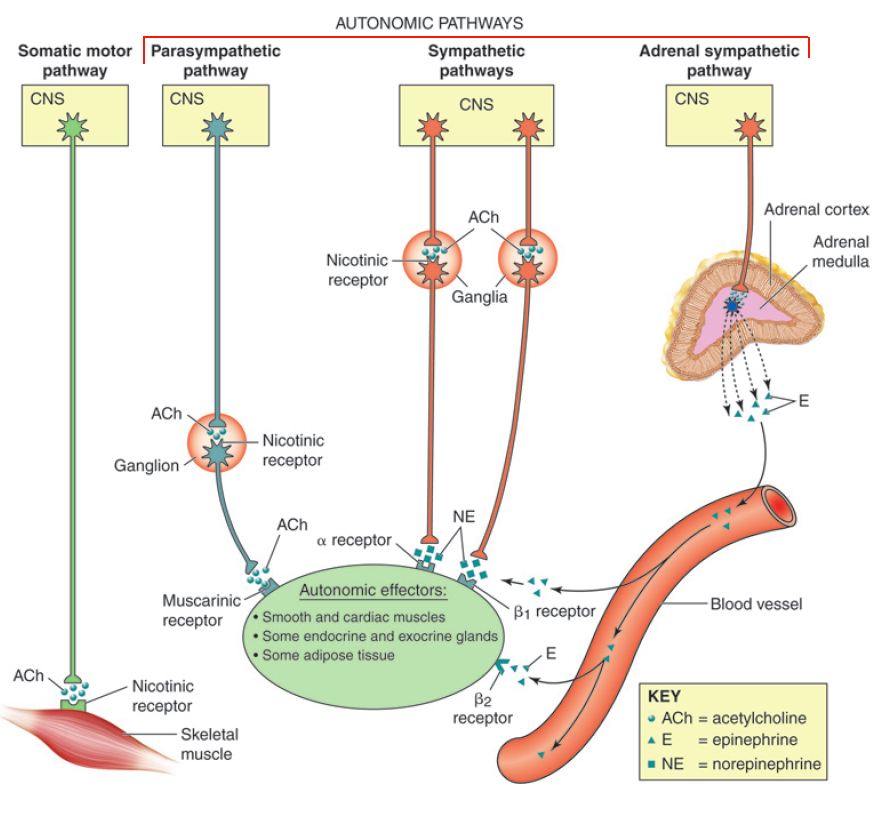
When we are faced with a situation that demands our earnest attention – that is, whether to “fight or flee,” our brain needs to receive as much oxygen as it can get (for processing sensory information); our respiratory passageways need to open up for moving as much air as possible; and the heart needs to pump more blood to move oxygen to the needed organs (brain and skeletal muscles). When we are faced with such a situation, it is unlikely that we will have time to eat (or even feel like eating), nor will we have the time to waste on going to the bathroom; hence, these functions will not be facilitated.
Within the autonomic nervous system, the sympathetic (“fight or flight”) and the parasympathetic (“rest and digest”) functions may be viewed as opposing each other. In general, the parasympathetic branch tends to exert an inhibitory effect on the target cells, while the sympathetic branch has an excitatory effect. Most organs, therefore, are innervated by both these branches of the autonomic nervous system to facilitate maintenance of homeostatic balance. As we learn more about these two systems, their modes of action will be clearer. The following table summarizes the major parasympathetic and/or sympathetic physiological effects on target cells, glands, and muscles. Note that many organs are dually innervated, while others are innervated only by the sympathetic branch. Blood vessels of abdominal viscera, skin of the limbs, and skeletal muscles are constricted by sympathetic nervous system activation; blood vessels are minimally impacted by activation of the parasympathetic nervous system except for certain areas of the body such as the blood vessels of the face that are associated with blushing.
| Organ | Sympathetic Effect | Parasympathetic Effect |
|---|---|---|
| Pupil | Dilation | Constriction |
| Lens | Far focus (lower curvature) | Near focus (increased curvature) |
| Salivary Gland secretion | High in viscosity | Serous |
| Heart | Increased rate and pressure | Lower rate and pressure |
| Lungs | Dilation of respiratory passages | Constriction of respiratory passages |
| Gastrointestinal | Decreased motility | Increased motility |
| Kidneys | Decreased filtration rate | Increased filtration rate |
| Male genitalia | Ejaculation | Erection |
| Vascular smooth muscle | Variable depending on the neurotransmitter | Relaxation |
| Sweat glands | Increased activity | No innervation |
| Arteries to skeletal muscle | Dilation | No innervation |
| Veins | Variable depending on the neurotransmitter | No innervation |
Homeostasis and Integrated Function
As noted previously, a balance of function in the autonomic nervous system is required to maintain a living organism. Activity in the autonomic nervous system is not a conscious endeavor that most people can normally control (however, with practice, autonomic nervous system can be controlled to some extent such as in deep meditation). Sympathetic and parasympathetic functions that maintain homeostatic balance and other internal body states are listed below.
Parasympathetic Functions:
- Stimulates visceral activity
- Decreases metabolic rate Decreases heart rate and blood pressure
- Conserves energy and promotes sedentary activities
- Increased salivary gland secretion (serous, and hence dilute)
- Increased digestive secretions
- Increased motility and blood flow in digestive tract
- Facilitation of urination and defecation.
Sympathetic Functions:
- Heightened mental alertness
- Increased metabolic rate
- Increased respiratory rate and dilation of respiratory passageways.
- Viscous saliva secretion (higher protein content, less water)
- Increased heart rate and blood pressure
- Energy reserves activated
- Reduced digestive and urinary functions
- Sweat glands activated
Dual Innervation
As you might expect, certain organs and tissues receive innervation from both the sympathetic and parasympathetic nervous systems. These organs are said to be “dually innervated.” An example of this is how the parasympathetic division facilitates micturition and defecation, while sympathetic input activates sphincters of the bladder and the rectum, impending micturition and defecation, respectively.
Everybody is familiar with asthma. Whatever the causality, asthma is characterized by bronchoconstriction due to increased sensitivity to irritating stimuli (which could be due to allergic response to certain antigens or due to irritants in the environment like coal dust, exhaust fumes, etc.). Increased mucus secretions rapid bronchoconstriction leads to labored breathing which we know as “asthma.”
Sympathetic and Parasympathetic Effectors
There are certain effectors in your body that are not dually innervated. Sweat glands, arrector pili muscles, adrenal medula, liver, adipocytes, lacrymal glands, radial muscle of the iris, juxtaglomerular apparatus, uterus and most vascular smooth muscles have only sympathetic innervation. In contrast to the sympathetic system, there are relatively few organs that function only with parasympathetic stimulation. Examples of such organs are the circular muscle of iris which causes pupillary constriction and the parietal cells of the stomach that secrete gastric acid.
Most vasculature of smooth muscles receive only sympathetic innervations. The balance of vascular constriction and dilation is mediated by sympathetic tone. The tone of a single vessel is proportional to the sympathetic stimulation it is receiving. More stimulation leads to more constriction, and, as sympathetic input to the vessel decreases, the vessel relaxes. Also, it is important to recognize that the impact of the release of epinephrine or norepinephrine on blood vessels is dependent on the subtype of neurotransmitter receptor on those blood vessels. For example, sympathetic activation leads to constriction of most vasculature in the body (mediated through stimulation of alpha-type receptors), but leads to dilation of coronary vessels supplying the heart (mediated through stimulation of beta-type receptors). The difference in how the vessel responds is due to the type of adrenergic receptor, and, ultimately, what type of intracellular signaling pathways are engaged to drive the vascular response. Adrenergic receptors are stimulated by either norepinephrine or epinephrine.
Most vessels in the body have the alpha-type receptor for binding norepinephrine, which results in vasoconstriction. Vasculature of the heart, on the other hand, has beta-type receptors for binding norepinephrine, which results in vasodilation. Also, recall that sympathetic nervous system activation causes release of epinephrine by chromaffin cells of adrenal medullae. Epinephrine has a greater effect on stimulating beta receptors than does norepinephrine, which means that epinephrine has a stronger effect on cardiac stimulation and a much weaker effect on blood vessels in muscles. Stimulation of beta receptors in the heart causes dilation of coronary blood vessels supplying the heart, increase heart rate, and increase in the strength of contraction of cardiac muscle. Blood vessels in skeletal muscles express alpha and beta adrenergic receptors. During exercise, blood vessels in skeletal muscle dilate, and, among many possible factors (ATP, lactic acid, carbon dioxide, oxygen, adenosine) that could cause this vasodilation, circulating epinephrine released into the bloodstream by chromaffin cells in the adrenal medullae is one other possible factor.
Nervous Levels of Organization Review
Neurons are considered the simplest functional unit of nervous tissue. The soma (cell body) is the region of the neuron that integrates all the incoming information from the dendrites. On one side of the soma are short, tapering processes called dendrites (Greek, dendreon – tree). Most neurons have many, highly branched dendrites, although they may have as few as one. Dendrites receive information from other neurons and transfer it to the cell body. The soma is similar to the dendrites in that it can also receive inputs from other neurons. The incoming information is coded as electrical signals that are integrated to determine what, if any, response is required. If the incoming signal is large enough, it travels to the process on the other side of the soma called the axon. The axon functions like a cable, relaying electrical signals away from the cell body towards other neurons or cells (e.g. muscles, glands). The axon may travel to its target as a single fiber, but some axons form branches called collaterals, so that they can interact with not just one, but many target cells.
Neurons are classified according to 2 different characteristics: their morphology (anatomical or structural features) and their function. Sensory (afferent) neurons are specialized for detection of sensory information (e.g. light, pressure, vibration, temperature, chemicals, etc.). They transduce physical and chemical stimuli into electrical signals and transfer this information from the periphery towards the central nervous system for processing. Interneurons (association neurons) are located entirely within the central nervous system (with the dendrites, soma and axons of the cell all residing within the CNS). Interneurons are also referred to as association neurons, in part because they are sandwiched between sensory and motor neurons where they integrate and distribute sensory information and coordinate motor output. Motor (efferent) neurons carry impulses or motor commands away from the central nervous system to effectors/target organs (e.g. muscles and glands). Most motor neurons have dendrites and cell bodies in the CNS and axons that exit the CNS to form peripheral nerves that travel to effectors (targets). Most neurons are surrounded by glial cells (neuroglia), the other cell type found in the nervous tissue. Glial cells are the supportive cells of the nervous system and are 10 times more numerous than neurons.
Consider again the learning objectives for this module. Could you demonstrate each of these objectives? If not, consider reviewing content related to these objectives.
- Classify the organs that are part of the nervous system as belonging to the central nervous system (CNS) or the peripheral nervous system (PNS).
- Within a neuron, identify the soma, axon and dendrite and describe the main function of each region.
- Identify neurons based on anatomical features: unipolar, bipolar, multipolar and anaxonic and based on functional properties: sensory, motor, interneuron.
- List the four types of CNS glial cells and describe their function.
- List the two types of PNS glial cells and describe their function. Describe the anatomical relationship between the glial cells and the PNS.
- Compare the structure of myelinated vs. unmyelinated axons. Distinguish between white matter and gray matter.
- Describe the transmembrane potential or voltage across the cell membrane and how it is measured.
- Contrast the relative concentrations of ions in body solutions inside and outside of a cell (sodium, potassium, calcium and chloride ions).
- Explain how four factors determine a neuron’s resting membrane potential.
- Explain action potential.
- Identify the presynaptic and postsynaptic cells at a synapse.
- Explain synaptic transmission in terms of the structural and functional features of electrical and chemical synapses.
- Explain how a single neurotransmitter may have different effects at different postsynaptic cells.
- Identify the four classes of neurotransmitters and identify the most common excitatory and inhibitory neurotransmitters.
- Explain the role of the autonomic nervous system as a motor division of the nervous system.
- Compare the somatic and autonomic nervous systems.
- Contrast the anatomy of the parasympathetic and sympathetic systems.
- Describe major parasympathetic and sympathetic physiological effects on target organs.

