Chapter 12: Estimating Age in Human Skeletal Remains
ESTIMATING AGE
Estimating the age-at-death of an individual is an important step in both forensic and bioarcheological studies as it contributes to the demographic profile of an individual and population in forensic and archaeological contexts. In addition, it offers an appreciation for the regional and temporal variation in the aging process. In forensic cases, estimating the age-at-death is crucial as it can directly contribute to a positive identification.4 Age is one of the pieces of information that can be impute into missing persons databases and further narrow down a list of missing person. Several of the subadult methods mentioned in this chapter can also be used to determine if a living individual is an adult or juvenile, which could be used to decide if someone is prosecuted as a adult or not.1
Estimating age from the skeleton relies on the measurement of two basic physiological processes: (1) growth and development and (2) degeneration (or aging). From fetal development on, our bones and teeth grow and change at a predictable rate. This provides for relatively accurate age estimates. After our bones and teeth cease to grow and develop, the bone begins to undergo structural changes, or degeneration, associated with aging. This does not happen at such predictable rates and, therefore, results in less accurate or larger age-range estimations.2
Estimating Age in Subadults
During growth and development stages, three primary methods used for estimations of age of subadults (those under the age of 18) are epiphyseal union, bone growth/development,and dental development.1/2
Bone Growth and Development
During the early stages of development, when there is little dental development, the method most commonly used for age estimation is diaphyseal length. This method involves measuring the length of long bone shafts which is strongly correlated with age.1 Bone measurements exist for fetal material through approximately six years of age.6 However, this method is most commonly used for fetal material.1
Long bones, as will as several other bones of the body, develop in at least two ossification centers. The primary center of ossification in long bones is the diaphysis and the secondary centers are the epiphyses. The formation and ossification of these different centers can also be useful in ageing subadult remains.1
Epiphyseal Union
Epiphyseal union, or epiphyseal fusion, refers to the appearance and closure of the epiphyseal plates between the primary centers of growth in a bone and the subsequent centers of growth. Prior to complete union, the cartilaginous area between the primary and secondary centers of growth is also referred as the growth plates.2 Because of this a child may have up to 600 separate bones, but by adulthood only 206.6 Different areas of the skeleton have documented differences in the appearance and closure of epiphyses, making this a reliable method for aging subadult remains.2 Epiphyseal union can be extremely helpful in aging if no dentition is present or if you are working with a commingled burial.6
As an example of its utility in the identification process, epiphyseal development was used to identify two subadult victims of a fatal fire in Flint, Michigan, in February 2010. The remains represented two young girls, ages three and four. Due to the intensity of the fire, the subadult victims were differentiated from each other through the appearance of the patella, the kneecap. The patella is a bone that develops within the tendon of the quadriceps muscle at the knee joint. The patella begins to form around three to four years of age. In the example above, radiographs of the knees showed the presence of a patella in the four-year-old girl and the absence of a clearly discernible patella in the three-year-old.2 See Table 1 below for when different bones ossify and fuse.
Table 1: Age Determination by Bone Ossification and Fusion
|
Characteristic |
Age estimation |
|
Metopic suture fusion |
Closure by 1–2 years, fusion by 7–8 years |
|
Lateral portion of occipital bone fusion with squamous bone |
1–3 years |
|
Basioccipital fusion with occipital |
5–7 years |
|
Fusion of occipital with sphenoid |
11–16 years (in females), 13–18 years (in males) |
|
Mandibular symphysis fusion |
6–8 months |
|
Medial clavicular epiphysis |
Mid-twenties or 15–35 years |
|
Coracoid Process in scapula |
15–17 years |
|
Glenoid epiphysis |
17–18 years |
|
Acromial epiphysis |
20 years |
|
Ribs’ head epiphysis |
17–25 years |
|
Segment 3 and 4 of sternum |
4–10 years |
|
Segment 2 with 3–4 of sternum |
11–16 years |
|
Segment 1 with 2–3–4 of sternum |
15–20 years |
|
Xiphoid to body |
40+ years |
|
Xiphoid appearance |
3–6 years |
|
Transverse lines (S1 and S2) in sacrum |
Mid-twenties or later5 |
|
Patella Ossifies |
3-4 years2 |
|
Humerus distal epiphysis |
11–15 years female, 12–17 years male5 |
|
Humerus proximal epiphysis (Head) |
13–17 years female, 16–20 years male5 |
|
Radial distal epiphysis |
11–13 years female, 14–17 years male5 |
|
Radial proximal epiphysis |
14–17 years female, 16–20 years male5 |
|
Ulna proximal epiphysis fused |
19 years of age6 |
|
Ulna distal epiphysis fused |
17-20 years of age6 |
|
Ischiopubic ramus |
5–8 years5 |
|
Acetabulum |
11–17 years5 |
|
Ischial tuberosity |
16–20 years5 |
|
Iliac Crest |
Fused by age 236 |
|
Sacrum |
Fuses from inferior to superior between the ages of 18-25 6 |
|
Femoral distal epiphysis |
14–18 years female, 16–20 years male5 |
|
Femoral head fusion |
12–16 years female, 14–19 years male5 |
|
Greater Trochanter |
14-19 years6 |
|
Tibial distal epiphysis |
14–16 years female, 15–18 years male5 |
|
Tibial proximal epiphysis |
13–17 years female, 15–19 years male5 |
|
Fibula proximal epiphysis fused |
14-22 years of age6 |
|
Fibula distal epiphysis fused |
11-20 years of age6 |
Table 1 Age determination in male and female using ossification and epiphyseal union.5

Dental Development
Dental development begins during fetal stages of growth and continues until the complete formation and eruption of the adult third molars (if present). The first set of teeth to appear are called deciduous or baby teeth. Individuals develop a total of 20 deciduous teeth, including incisors, canines, and molars. These are generally replaced by adult dentition as an individual grows. A total of 32 teeth are represented in the adult dental arcade, including incisors, canines, premolars, and molars. When dental development is used for age estimations, researchers use both tooth-formation patterns and eruption schedules as determining evidence. For example, the crown of the tooth forms first followed by the formation of the tooth root. During development, an individual can exhibit a partially formed crown or a complete crown but a partially formed root. The teeth generally begin the eruption process once the crown of the tooth is complete.2 Radiographic examination of the dentition is needed to show the development of the crown, root, and closure of the root.6 The developmental stages of dentition are one of the most reliable and consistent aging methods for subadults.2 However, despite each tooth having a general age of eruption, these eruption times can vary from a few months to a few years as each child develops along a different trajectory.6 See Tables 2 and 3 below for when different teeth erupt.
Table 2: Tooth Eruption to age 3
|
Tooth |
Complete Eruption |
|
Upper Central Incisor |
10.5 Months |
|
Upper Lateral Incisor |
1.5 yrs. |
|
Upper Canine |
2.5 yrs. |
|
Upper 1st Molar |
1.5 yrs. |
|
Upper 2nd Molar |
2.5 yrs. |
|
Lower Central Incisor |
10.5 Months |
|
Lower Lateral Incisor |
1.5 yrs. |
|
Lower Canine |
2.5 yrs. |
|
Lower 1st Molar |
1.5 yrs. |
|
Lower 2nd Molar |
2.5 yrs.1 |
Table 2 Age estimation using eruption of deciduous teeth. All deciduous teeth should be in by age 3.1
Table 3: Tooth Eruption of Adult Teeth
|
Tooth |
Complete Eruption |
|
Upper Central Incisor |
7.5 yrs. |
|
Upper Lateral Incisor |
9.5 yrs. |
|
Upper Canine |
12.5 yrs. |
|
Upper 1st Premolar |
11.5 yrs. |
|
Upper 2nd Premolar |
12.5 yrs. |
|
Upper 1st Molar |
6.5 yrs. |
|
Upper 2nd Molar |
13.5 yrs. |
|
Upper 3rd Molar (Wisdom Tooth) |
20.5 yrs. |
|
Lower Central Incisor |
7.5 yrs. |
|
Lower Lateral Incisor |
7.5 yrs. |
|
Lower Canine |
11.5 yrs. |
|
Lower 1st Premolar |
11.5 yrs. |
|
Lower 2nd Premolar |
12.5 yrs. |
|
Lower 1st Molar |
6.5 yrs. |
|
Lower 2nd Molar |
12.5 yrs.1 |
|
Lower 3rd Molar (Wisdom Tooth) |
18-21 yrs.6 |
Table 3 Age estimation using eruption of adult teeth.1
Estimating Age in Adults
Changes to the Pubic Symphysis and Sternal Rib Ends
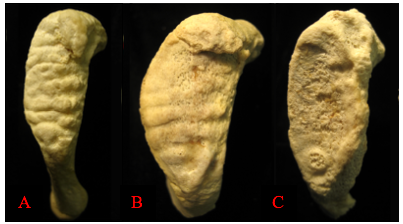
Degenerative changes in the skeleton typically begin after 18 years of age, with more prominent changes developing after an individual reaches middle adulthood (commonly defined as after 35 years of age in osteology). These changes are most easily seen around joint surfaces of the pelvis, the cranial vault, and the ribs. The pubic symphysis surfaces of the pelvis and the sternal ends of the ribs show metamorphic changes from young adulthood to older adulthood. The pubic symphysis is a joint that unites the left and right halves of the pelvis. The surface of the pubic symphysis changes during adulthood, beginning as a surface with pronounced ridges (called billowing) and flattening with a more distinct rim to the pubic symphysis as an individual ages. As with all metamorphic age changes, older adults tend to develop lipping around the joint surfaces as well as a breakdown of the joint surfaces. The most commonly used method for aging adult skeletons from the pubic symphysis is the Suchey-Brooks method. This method divides the changes seen with the pubic symphysis into six phases based on macroscopic age-related changes to the surface. Figure 12.1 provides a visual of the degenerative changes that typically occur on the pubic symphysis.2
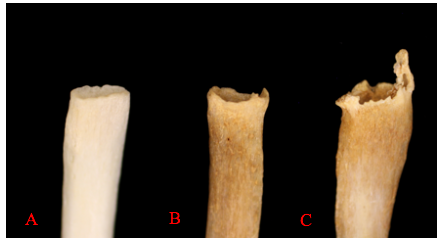
The sternal end of the ribs, the anterior end of the rib that connects via cartilage to the sternum, is also used in age estimations of adults. This method, first developed by M. Y. İşcan and colleagues, looks at both the change in shape of the sternal end but also the quality of the bone. The sternal end first develops a billowing appearance in young adulthood. The bone typically develops a wider and deeper cupped end as an individual ages. Older adults tend to exhibit bony extensions of the sternal end rim as attaching cartilage ossifies. Figure 12.2 provides a visual of the degenerative changes that typically occur in sternal rib ends.2
Auricular Surface
The Auricular surface is where the ilium forms a joint with the sacrum. Just like the Pubic symphysis it shows changes with age. With increasing age the horizontal lines present across the surface or billows fill in until they can no longer be seen. This joint surface also becomes more porous with time. Initially there will be no porosity, then small holes will form which will get bigger with time. Bony spicules can also form in the muscle attachment site just above this joint and the texture of the surface can become denser with age. Finally, a rim can form around the auricular surface. These changes to the joint can be tracked across six phases which allows age to be determined.1
Dental Wear
Dental wear or attrition begin as the teeth erupt into the mouth. As an individual chews the topmost layer of tooth is ground off. As an individual ages, the amount of wear increases first through the enamel and then to the dentine.6 Most of this wear occurs on the occlusal surface and, because it is irreversible, wear increases with age.7 Individuals of advanced age or those eating course foods may wear the teeth to the point the crown is absent, and the root then becomes the masticatory surface. Due to the variability in the texture of foods eaten, dental wear may make an individual appear older or younger. For example, modern populations consuming extremely soft foods will show a much-reduced dental wear. Other activities may also be responsible for tooth wear including bruxism and using the teeth as tools. Prehistoric tool use included working hides and doing light retouch on lithics, while today teeth may be used as tools to open packaging. Brothwell developed a general system for dental wear that is helpful in aging older individuals.6
Wear is most regular on the molar teeth, so it is these that are usually the focus of study. The rate of wear on the molars in a particular population under study may be estimated by comparing wear on the different molar types using the difference in eruption times between the first, second and third molars as a means of calibration. It is based upon the generation of a baseline in which wear is observed in juveniles for whom age can be reliably assessed from dental development. The first, second and third molars erupt at approximately 6, 12, and 18 years respectively. The age of the child, minus the eruption time of the tooth, gives the functional age of the tooth – the number of years that it has been in occlusion. The amount of wear observed can therefore be related to the number of years for which the molar has been in occlusion. For example, when the second molar erupts, the wear on the first molar represents about six years’ worth of wear; when the third molar erupts, the first molar shows ca. 12 years of wear, and the second will have a functional age of about six years. Among these, if (using comparison with molars in the baseline group) a second molar on an individual shows a functional age of about 12 years, and a third molar about six years then that individual is probably about 24 years old; by looking at their first molars we can gauge the amount of wear that represents ca. 18 years of tooth function. Those with second molars with functional ages of about 18 years will be aged about 30 at death. By this process of successive extrapolation age can be estimated throughout the adult cohort, starting with the youngest and progressing to those with the most heavily worn dentition.7
When molar loss becomes advanced, which is often the case once individuals are more than about 50 years of age, molar wear can no longer be readily applied as an ageing method. That it is not easily applicable in the second half of the potential human lifespan is something dental wear holds in common with other skeletal adult ageing methods. Following tooth loss, there is localized resorption of alveolar bone, which is normally irreversible and may be lifelong. Among those showing tooth loss, an inverse relationship between mandibular alveolar bone height and age has been reported in living and skeletal populations. This potentially offers a means of estimating age in mandibles where adequate documentation of molar wear is precluded by ante mortem tooth loss, but there are difficulties in operationalizing these observations into providing a practical age-estimation method.7
Suture Closure
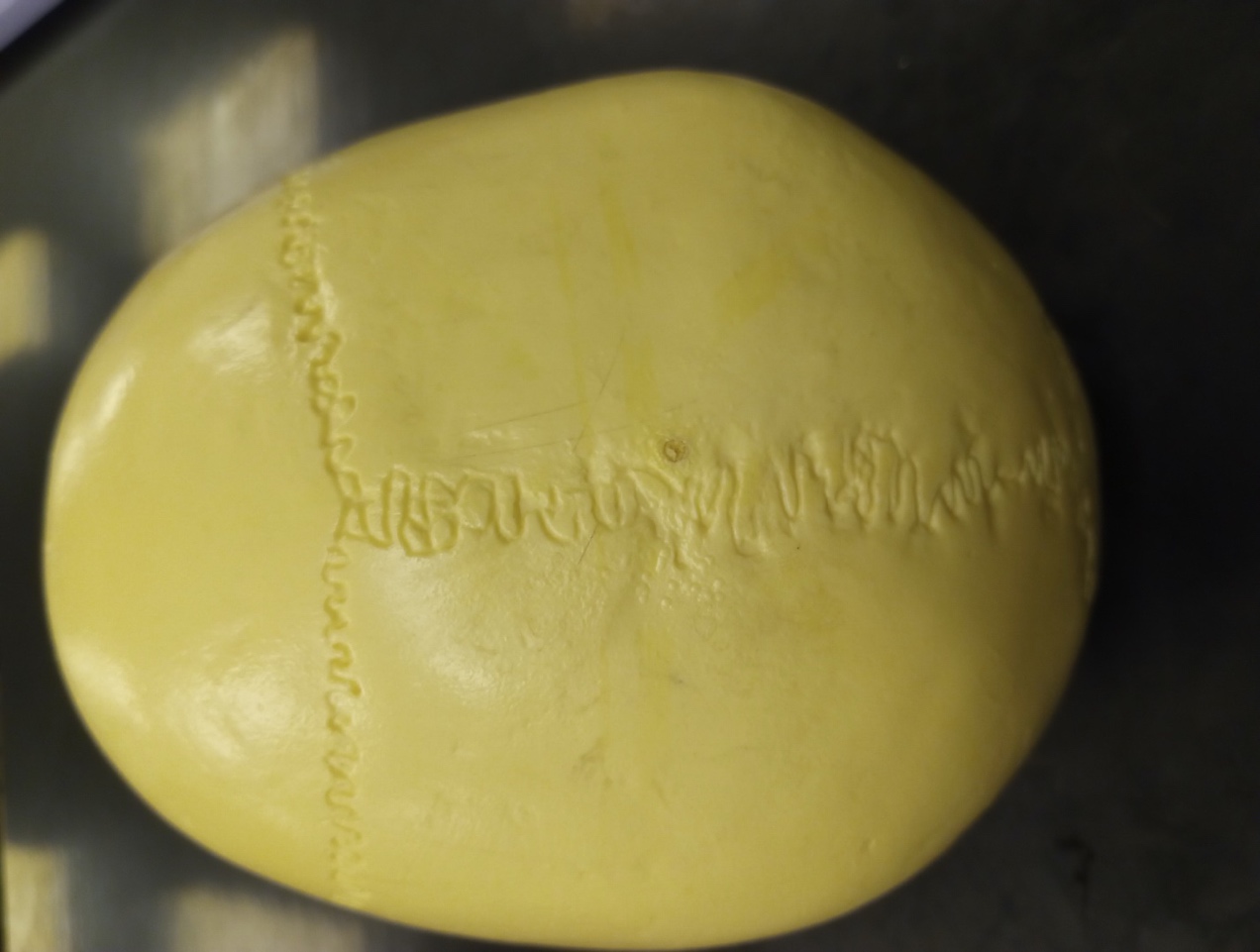
Cranial suture closure may also be used to age adults, but in general will provide broad ranges into which a specimen will fall. Sutures should be recorded as unobservable, open (no evidence of closure), minimal closure (up to 50% closure observed), significant closure (mostly fused, but not complete), complete obliteration (totally fused).6 If all sutures are completely closed this suggest the individual is quite elderly.1

Degenerative Changes
Degenerative change in the post cranium is similar to that seen in the articulation sites in the skull, with osteophytosis and erosion.6 Osteoarthritis can lead to the breakdown of the cartilage which covers and protects the joint surface. This can cause osteophytes around the margins of the joint surface. The likelihood of having osteoarthritis increases with age.1
Osteophyte formation can also occur on the vertebral column and the likelihood increases with age, and the development of vertebral osteophytes has been shown to be a general indicator of age. Osteophytes can be defined as “abnormal bony growths or bone spurs that are bony projections formed along joints.” Although osteophyte formation can occur on almost any degenerating joint, such as the hip, knee, and distal interphalangeal joints, they are most common on the vertebrae. Three types of osteophytes can be distinguished. First, traction spurring, which occurs at the point of tendon and ligament insertion. Second, inflammatory spurring, where the ossification of a syndesmophyte originates from the intervertebral discs and the spinal ligaments. This occurs, for example, in ankylosing spondylitis. A syndesmophyte can be defined as paravertebral ossification that is usually orientated vertically, whereas the osteophyte is usually orientated horizontally. And finally, the genuine osteophyte that arises in the periosteum overlying the bone at the junction between the cartilage and the bone. The bony outgrowths occur in various stages. Initially starting small, bony outgrows eventually become bigger and more severe. Ultimately, it can become so severe that it can lead to ankylosis.4
Previous studies—conducted on African-Americans, Japanese, and Thai populations—have indicated that vertebral degeneration is a viable method of age-at-death estimation. However, the living conditions, social status, and occupations of the skeletal collections used in these studies all vary and can have a direct impact on osteophyte growths. Additionally, because age-at-death estimation via osteophyte formation is thought to be population-specific, it is important to use the same populations when conducting a estimation. Regression equations have developed for each section of the vertebral column and for the combined sections, to estimate age-at-death from the degree of osteophyte formation.4
The study by Van der Merwe et al. looks at the different regions of the vertebral column in relation to osteophyte formation and concludes that the thoracic part of the vertebral column shows the least osteophyte formation in contrast to the cervical and lumbar parts. This can be explained by the expectation that more osteophyte formation will occur in the cervical and lumbar region because the regions are more mobile and weight-bearing. The cervical region is the most mobile region, and in addition, this region is responsible for supporting head-bearing and shoulder movement. The thoracic region guides the ribs and internal organs but is not overloaded, resulting in less osteophyte formation. The lumbar portion, on the other hand, carries the most weight since this region is located at the bottom and therefore has to bear most of the thorax. It is therefore considered that the lumbar portion is under more pressure than the other regions, which can also be seen in the osteophyte pattern.4
A study by Snodgrass, showed that in females there was more variation in osteophyte score and age-at-death, in contrast to males. This finding may be explained from a biological perspective in that males participate in more strenuous activities at a younger age as opposed to females (i.e. sexual division of labor). This results in higher levels of strain on the vertebral column, which can lead to more and severe osteophyte formation in later life. On the other hand, an explanation for this finding may be that males are generally heavier and more robust than females, causing in more weight on the vertebral column, which also promotes osteophyte formation. The importance of socio-economic and physical activities in the light of the different sexes and between different populations should also be highlighted, as this is important for the forensic use of age markers, which can also be influenced by physical activity.4
DNA
Epigenetics is a large area of research nowadays. The main epigenetic features (histone modifications, regulation by non-coding RNAs and DNAm) have been associated with several clinical conditions, such as cancer and Alzheimer’s disease, and forensic issues, including age estimation. One of the most important bases of aging research is DNAm, which has arisen in recent years as one of the most promising and investigated epigenetic features associated with aging. DNAm is characterized by the addition of a methyl group (CH3) to the fifth carbon (5C) position of cytosines in the DNA molecule. During aging, there is a change in the human genome methylation levels: some genes across the genome lose methylation, and some gain methylation. Based on these consistent alterations in DNAm levels of some genes (age-correlated genes), the first generation of “epigenetic clocks” has arisen in different tissue types.3
Despite the growth in DNAm age research and the great development of many tissue-specific APMs and some multi-tissue APMs, there are several confusing factors that should be considered before the implementation of DNAm age as a new tool for forensic casework. DNAm levels can be affected by intrinsic influences (such as aging, sex, or ancestry) or environmental factors (including lifestyle, disease, alcohol consumption, or social environments). It should also be noted that the same genes or CpGs reveal different patterns of age correlation in different tissues, for instance blood vs bone samples.3
Conclusion
Several methods used for the estimation of age has their advantages and disadvantages. No aging technique is even close to 100% accuracy. There are two sources of this fault: (1) individual differences and (2) differences between the sample population and the population of origin. No aging methods should be used alone unless there is no choice. Choice of method is, of course, limited when partial or fragmentary remains are the only material accessible. However, even in these cases always offer a range when estimating age. It is far better to include a 10- to 12-year age range, particularly in big individuals, and be successful in matching the lost person by other individuality than to give a 3- to 5-year range and miss the recognition entirely.5
References:
1. Angi M. Christensen, Nicholas V. Passalacqua, and Eric J. Bartelink, Forensic Anthropology: Current Methods and Practice, 2nd ed. (London: Academic Press, 2019): 38, 307-342.
2. Ashley Kendell, Alex Perrone, and Colleen Milligan, “Bioarcheology and Forensic Anthropology” In Explorations, ed. Beth Shook, Katie Nelson, Kelsie Aguilera and Lara Braff (Arlington: American Anthropological Association, 2019). https://pressbooksdev.oer.hawaii.edu/explorationsbioanth/chapter/osteology/.
3. Helena Correia Dias, Eugénia Cunha,Francisco Corte Real, and Licínio Manco, “Challenges and (Un)Certainties for DNAm Age Estimation in Future,” Forensic Science 2 (2022): 601-614.
4. Iris F. Sluis, Bjørn P. Bartholdy, Menno L.P. Hoogland, and Sarah A. Schrader, “Age estimation using vertebral bone spurs; Testing the efficacy of three methods on a European population,” Forensic Science International: Reports 6 (2022). https://www.sciencedirect.com/science/article/pii/S2665910722000470.
5. Purva Wagisha Upadhyay and Amarnath Mishra, “Forensic Anthropology” in Biological Anthropology – Applications and Case Studies, ed. Alessio Vovlas (London: IntechOpen, 2021). https://www.intechopen.com/chapters/733723
6. Roberta Hall, Kenneth Beals, Holm Neumann, Georg Neumann, and Gwyn Madden, Introduction to Human Osteology (Michigan: Grand Valley State University, 2010). https://pressbooks.gvsu.edu/introhumanosteology/.
7. Simon A.Mays, SoniaZakrzewski, S.Field, “The relationship between dental wear and age at death in British archaeological human skeletal remains: A re-evaluation of the ‘Brothwell chart’,” Journal of Archaeological Science: Reports 46 (2022). https://www.sciencedirect.com/science/article/pii/S2352409X22003704.
Figure Attributions:
Figure 12.1 Original to Introduction to Forensic Anthropology
Figure 12.2 Original to Introduction to Forensic Anthropology
Figure 12.3 Original to Introduction to Forensic Anthropology
Figure 12.4 Original to Introduction to Forensic Anthropology
Figure 12.5 Example of the progression of degenerative changes to the pubic symphysis a derived work by Ashley Kendell original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License. [Original photos by Dr. Julie Fleischman used by permission.]
Figure 12.6 Examples of degenerative changes to the sternal rib end by Alex Perrone original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
Figure 12.7 Original to Introduction to Forensic Anthropology
Figure 12.8 Original to Introduction to Forensic Anthropology

