6.7 Human Responses to Extreme Climates
Figure 6.7.1 Men from Maasai Mara, Kenya.
Built for Heat
These tall, slender men live near the equator in Kenya, East Africa — one of the hottest regions of the world. These and the other people of his tribal group, called the Maasai, are among the tallest, most linear people on the planet. Their body build is thought to be an adaptation to their climate, which is hot year-round.
Climate Extremes
Climate refers to the average weather conditions in a region over a long period of time. One of the main determinants of climate is temperature. Both hot and cold temperatures are serious environmental stresses on the human body.
In the cold, there is risk of hypothermia, which is a dangerous decrease in core body temperature. The normal temperature of the human body is 37 degrees C (98.6 degrees F). Hypothermia sets in when body temperature drops to 34.4 degrees C (94 degrees F). If body temperature falls below 29.4 degrees C (85 degrees F), it starts to cool very rapidly because the body’s temperature regulation mechanism starts to fail.
The opposite problem occurs in the heat, where the risk is hyperthermia, which is a dangerous increase in core body temperature. If human body temperature rises above about 40.6 degrees C (105 degrees F), hyperthermia may become life threatening. If a temperature this high persists more than a few days, it generally damages the brain and other internal organs, leading to death.
Human Adaptation to Heat and Cold
Humans are the most widespread species on the planet, and they have lived in extreme climates for tens of thousands of years. As a result, many human populations have had to cope with extreme temperatures for hundreds of generations, which has forced them to develop genetic adaptations to these climate extremes.
The size and proportions of the human body may play an important role in how well an individual is able to handle hot or cold temperatures. In general, people with a tall, slender build, like the Maasai man pictured in Figure 6.7.1, are well adapted to heat, whereas people with a short, stocky build (like the Indigenous North American Inuit pictured in Figure 6.7.2) are well adapted to cold. These relationships between body build and climate were first noticed in other animal species in the 1800s by biologists Carl Bergmann and Joel Allen. These scientists formulated what are now known as Bergmann’s and Allen’s rules.
Figure 6.7.2 Indigenous North American Inuit.
Bergmann’s Rule
Bergmann's rule states that within a broadly distributed taxonomic group, populations or species of larger size are found in colder environments, whereas populations or species of smaller size are found in warmer environments. Bergmann’s rule has been shown to generally apply to widespread species of mammals and birds, although there are also many exceptions to the rule.
What explains Bergmann’s rule? Larger animals have a lower surface area to volume ratio than smaller animals, which is illustrated in Table 6.7.1 for a simple shape, a cube. From the table, you can see how the surface area to volume ratio of a cube decreases dramatically as the size of the cube increases. Because heat is lost through the surface of the body, an animal with a smaller surface area to volume ratio radiates less body heat per unit of mass. The larger body mass also allows the animal to generate more heat. A larger animal has more cells, so it can produce more body heat as a byproduct of cellular metabolism. Both of these factors allow a larger animal to stay warmer in a cold climate.
Table 6.7.1
Relationship of Surface Area to Volume in Cubes of Different Sizes
| Relationship of Surface Area to Volume in Cubes of Different Sizes | |||
| Side of Cube (cm) | Surface Area of Cube (cm2) | Volume of Cube (cm3) | Surface Area:Volume Ratio |
| 2 | 24 | 8 | 3:1 |
| 4 | 96 | 64 | 3:2 |
| 6 | 216 | 216 | 3:3 |
| 12 | 864 | 1728 | 3:6 |
| 20 | 2400 | 8000 | 3:10 |
Warmer climates impose the opposite problem: body heat generated by metabolism needs to be dissipated quickly rather than stored within the body. Smaller animals have a higher surface area to volume ratio that maximizes heat loss through the surface of the body and helps cool the body. With less mass and fewer cells, smaller animals also generate less heat due to cellular metabolism.

Anthropologists have found that many human populations tend to follow Bergmann’s rule. For example, a study of 100 human populations in the 1950s found a strong negative correlation between mean body mass and average yearly temperature. In other words, higher body mass was generally found in colder places, and lower body mass was generally found in hotter places.
There are also exceptions to the rule, in part because we use cultural responses to temper environmental stresses so we do not need to change genetically or physiologically in order to cope. Humans, for example, use clothing and heated buildings to stay warm in cold climates, which tends to counter the effects of natural selection changing human body shape in cold climates.
Allen’s Rule
Allen’s rule is a corollary of Bergmann’s rule. It states that animals living in hotter climates generally have longer extremities (such as limbs, tails, snouts, and ears) than closely related animals living in colder climates. The explanation for Allen’s rule is similar to the rationale behind Bergmann’s rule. Longer extremities maximize an animal’s surface area, allowing greater heat loss through the surface of the body. Therefore, having long extremities is adaptive in hot climates where the main challenge is dissipating body heat.
Anthropologists have noted that, in populations that have lived in tropical regions for long periods of time, the limbs of people tend to be longer in proportion to overall body height. The Maasai man pictured in Figure 6.7.1 is a clear example. His exceptionally long limbs — like those of other members of his population — are optimally proportioned for the hot climate in Kenya. The shorter-limbed body proportions of the Inuit people (Figure 6.7.2) suit them well for their cold climate. Marked differences in limb length have also been observed in related populations that have lived for long periods of time at different altitudes. High altitudes have colder climates than lower altitudes and — consistent with Allen’s rule —people tend to have shorter limbs at higher altitudes.
Other Human Responses to Heat
Humans exhibit several other responses to high temperatures that are generally considered either short-term physiological responses or examples of longer-term acclimatization.
Sweating and Humidity

Because humans are basically tropical animals, we generally have an easier time dealing with excessive heat than excessive cold. Evaporation of sweat is the main way we cool the body. The dancer in Figure 6.7.4 is sweating copiously while working out in a hot environment. Why does sweating cool us? When sweat evaporates from the skin, it requires heat. The heat comes from the surface of the body, resulting in evaporative cooling.
How well we can deal with high air temperatures depends in large part on the humidity of the air. We have a harder time losing excess body heat when the humidity is high because our sweat does not evaporate as well as it does when the humidity is low. Instead, the sweat stays on the skin, making us feel clammy and warmer than we would feel if the humidity were lower. If the air is dry, on the other hand, sweat evaporates readily, and we feel more comfortable. For this reason, we are able to tolerate higher temperatures when the humidity is low. This is the basis of the common aphorism, “It’s not the heat, but the humidity.”
The heat index (HI) is a number that combines air temperature and relative humidity to indicate how hot the air feels due to the humidity. The heat index is also called “apparent temperature.” Figure 6.7.5 shows the heat index at different combinations of air temperature and relative humidity. As you can see, when the humidity is very high, even a 90-degree F (32 degrees C) temperature can be very dangerous.
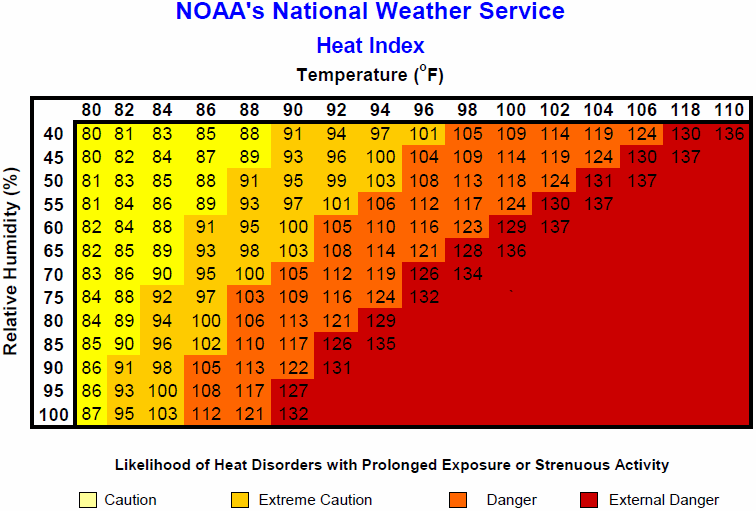
Acclimatization to Heat

If humidity is low, evaporation of sweat can be an effective way to keep the body from overheating. However, the loss of water and salts in sweat can also be dangerous. In very hot conditions, an adult may lose up to four litres of sweat per hour and up to 14 litres per day. Such water losses may cause severe dehydration if the water is not replaced by drinking much more than usual. The loss of salts may also upset the normal salt balance in the body, which can be dangerous. Becoming acclimatized to heat by gradually increasing the exposure time to high temperatures — particularly while exercising or doing physical work — can reduce the risk of these effects.
It may take up to 14 days to attain maximum heat acclimatization. As the body becomes acclimatized, sweat output increases, and sweating begins sooner. The salt content of the sweat also declines, as does the output of urine. These and other physiological changes help the body lose heat through the evaporation of sweat, while maintaining the proper balance of salts and fluids in the body. There may also be increased blood flow to the body surface through the widening of blood vessels near the skin. This is called vasodilation. This brings more heat from the body core to the skin, and from there it may be radiated out into the environment.
Becoming acclimatized to heat allows one to safely perform more exercise or work in the heat. It also helps prevent heat-related illnesses by reducing strain on the body. Heat-related illnesses — from least to most serious — include heat cramps, heat exhaustion, and heat stroke.
- Heat cramps are muscle spasms caused by loss of water and salts. They often follow prolonged sweating brought on by over-exertion in hot weather.
- Heat exhaustion is a condition in which over-heating of the body causes dizziness, headache, profuse sweating, rapid heartbeat. and other symptoms. Without prompt treatment, heat exhaustion can lead to heat stroke.
- Heat stroke is potentially life threatening and a medical emergency. Heat stroke results from prolonged exposure to high temperatures, usually in combination with dehydration. It leads to failure of the body’s temperature control system and is diagnosed when the core body temperature exceeds 105 degrees F (40 degrees C). Symptoms may include nausea, seizures, confusion, disorientation, and coma.
Acclimatization to heat, like other types of acclimatization, is a reversible process. Just as quickly as heat acclimatization occurs, the physiological changes fade away in the absence of heat exposure. The body returns to its baseline state within a week or two of no longer exercising or working at high temperatures.
Other Human Responses to Cold
Besides genetic difference in body build, there are two major ways the human body can respond to the cold. One way is by producing more body heat, and the other way is by conserving more body heat. An immediate response to cooling of the body is shivering. This is an involuntary and simultaneous contraction of many tiny muscles in the body. These muscle contractions generate a small amount of heat. Another early response to cold temperature is a narrowing of blood vessels near the skin. This is called vasoconstriction. This helps to shunt blood away from the body surface so more heat is held at the body core. The skin cools down and radiates less heat into the environment.
Hunting Response
At temperatures below freezing, vasoconstriction can be dangerous if it lasts too long. The extremities become too cold because of lack of blood flow, and cold injury (such as frostbite) may occur. Frostbite is tissue destruction that occurs when tissue freezes. You can see a mild-to-moderate case of frostbite of the fingers in Figure 6.7.7. If frostbite is severe, it may lead to gangrene and amputation of the affected extremities.
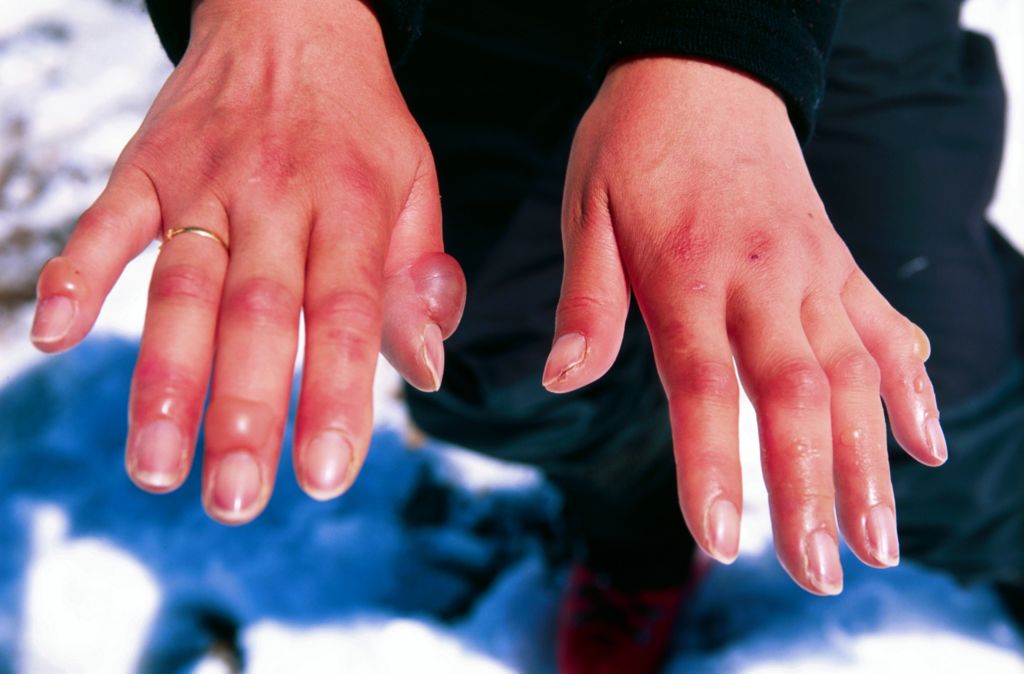
The body counters the possibility of cold injury with a reaction called the hunting response. This is a process of alternating vasoconstriction and vasodilation in extremities exposed to cold. About five to ten minutes after the start of cold exposure, the blood vessels in the extremities suddenly dilate, which increases blood flow and subsequently the temperature of the extremities. This is soon followed by another phase of vasoconstriction, and then the process repeats.
The hunting response occurs in most people, but several factors may influence the strength of the response. People who live or work regularly in cold environments show an increased hunting response. Through acclimatization, however, tropical residents can develop an increased response, which is indistinguishable from that of arctic residents. Genetic factors may play a role in the hunting response, but this is uncertain because it is difficult to differentiate between adaptation and acclimatization.
Persistent Vasoconstriction
Where temperatures rarely fall below freezing but are repeatedly very chilly, the hunting response may not occur. Instead, vasoconstriction may persist to keep heat within the body at the expense of cooling the skin. As long as the temperature stays above freezing, cold injury (such as frostbite) will not occur. This type of response has been shown to occur in indigenous desert dwellers in southern Africa and Australia, where the temperature is hot during the day and very cold at night. People in these populations also tend to deposit fat around the organs in their chest and abdomen. The fat serves as insulation, protecting vital structures from the cold.
High-Fat Diet
Besides shivering, another way to increase body heat is to raise the basal metabolic rate. The basal metabolic rate (BMR) is the amount of energy that a person needs to keep the body functioning at rest. The higher the BMR, the more heat the body generates, even without exercise or physical labor. The BMR can be increased by consuming large quantities of high-calorie fatty foods. People living in very cold subarctic regions, including the Inuit, traditionally ate whale and seal blubber and other high-fat foods, which helped them maintain a high BMR and stay warm.
Figure 6.7.8 Whale and seal blubber (mainly on abundant ring seals) is an important part of the traditional Inuit diet.
Feature: Human Biology in the News
Too many news stories report young children being seriously injured or dying from heat stroke in hot vehicles. On average, 38 children die in hot vehicles each year from heat-related deaths after being trapped inside. Most often, this happens by accident, when a parent or caregiver unknowingly leaves a sleeping child in a car. In other cases, children get into cars on their own, and then cannot get out again.
A child’s thermoregulatory system is not as efficient as that of an adult, and a child’s body temperature may increase as much as five times faster. This makes children prime candidates for heat stroke. A motor vehicle is also easily heated by direct sun. The windows of the vehicle allow solar radiation to pass through and heat up objects inside. A dark-coloured dashboard or seat may quickly reach a temperature of more than 180 degrees F (82 degrees C)! These hot surfaces can just as quickly heat the adjacent air, rapidly increasing the temperature of the air trapped inside the vehicle.
Here are several simple tips that parents and caregivers can follow to prevent heat stroke tragedies:
- Never leave children alone in or around cars — not even for a minute.
- Always open the back door and check the back seat before leaving your vehicle to be sure no child has been left behind.
- Put something you will need, such as your cell phone or handbag, in the back seat so you will have to open the back door to retrieve it whenever you park the car.
- Keep a large stuffed animal in the child’s car seat, and when the child is placed in the car seat, put the stuffed animal in the front passenger seat as a visual reminder that the child is in the back.
- Make sure you have a strict policy in place with everyone involved in the care of your child that you should always be called whenever your child does not show up at daycare or school as scheduled.
- Keep vehicles locked at all times, even in driveways and garages. Ask home visitors, child care providers, and neighbors to do the same.
- Keep car keys and remote vehicle openers out of reach of children.
- If a child is missing, immediately check the inside passenger compartments and trunks of all vehicles in the area. Check vehicles even if they are locked, because a child may lock a vehicle after entering and not be able to unlock it again to get out.
- If you see a child alone in a vehicle, call 911 immediately. If the child seems hot or sick, get them out of the vehicle as quickly as possible.
- Pay for gas at the pump and use drive-throughs at the bank, pharmacy, or wherever else they are available.
6.7 Summary
- Both hot and cold temperatures are serious environmental stresses on the human body. In the cold, there is risk of hypothermia, which is a dangerous decrease in core body temperature. In the heat, there is risk of hyperthermia, which is a dangerous increase in core body temperature.
- According to Bergmann’s rule, body size tends to be negatively correlated with temperature, because larger body size increases heat production and decreases heat loss. The opposite holds true for small body size. Bergmann’s rule applies to many human populations that are hot- or cold-adapted.
- According to Allen’s rule, the length of body extremities is positively correlated with temperature, because longer extremities are better at dissipating excess body heat. The opposite applies to shorter extremities. Allen’s rule applies to relative limb lengths in many human populations that have adapted to heat or cold.
- Sweating is the primary way that humans lose body heat. The evaporation of sweat from the skin cools the body. This only works well when the relative humidity is fairly low. At high relative humidity, sweat does not readily evaporate to cool us down. The heat index (HI) indicates how hot it feels due to the humidity.
- Gradually working longer and harder in the heat can bring about heat acclimatization, in which the body has improved responses to heat stress. For example, sweating starts earlier, sweat contains less salt, and vasodilation brings heat to the surface to help cool the body. Full acclimatization takes up to 14 days and reverses just as quickly when the heat stress is removed.
- The human body can respond to cold by producing more heat (by shivering or increasing the basal metabolic rate) or by conserving heat (by vasoconstriction at the body surface or a layer of fat-insulating internal organs).
- At temperatures below freezing, the hunting response occurs to prevent cold injury, such as frostbite. This is a process of alternating vasoconstriction and vasodilation in extremities that are exposed to dangerous cold. Where temperatures are repeatedly cold but rarely below freezing, the hunting response may not occur, and the skin may remain cold due to vasoconstriction alone.
6.7 Review Questions
- Compare and contrast hypothermia and hyperthermia.
- State Bergmann’s and Allen’s rules.
- How do the Maasai and Inuit match the predictions based on Bergmann’s and Allen’s rules?
- Explain how sweating cools the body.
- What is the heat index?
- Relate the heat index to evaporative cooling of the body.
- Identify three heat-related illnesses, from least to most serious.
- How does heat acclimatization occur?
- State two major ways the human body can respond to the cold, and give an example of each.
- Explain how and why the hunting response occurs.
- Define basal metabolic rate.
- How does a high-fat diet help prevent hypothermia?
- Explain why frostbite most commonly occurs in the extremities, such as the fingers and toes.
6.7 Explore More
What happens when you get heat stroke? – Douglas J. Casa, TED-Ed, 2014.
Hailstones’ Inupiaq Traditions | Life Below Zero, National Geographic, 2014.
How An Igloo Keeps You Warm, It’s Okay To Be Smart, 2017.
Why do we sweat? – John Murnan, TED-Ed, 2018.
Wim Hof Method, Wim Hof, 2011.
Attributions
Figure 6.7.1
- Maasai warrior by Ninaras on Wikimedia Commons is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/deed.en) license.
- Smiling man from Maasai Mara, Kenya by Sneha on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 6.7.2
- Inuit-Kleidung women by Ansgar Walk on Wikimedia Commons is used under a CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/deed.lv) license.
- Kulusuk, Tunumiit Inuit couple by Arian Zwegers on Wikimedia Commons is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/deed.en) license.
-
Inuit girls by Susan van Gelder on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
Figure 6.7.3
Bergmann’s_rule_-_Canis_lupus by Dhaval Vargiya at Yellowstone National Park on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 6.7.4
Sweating [photo] by Avi Richards on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 6.7.5
Heat_Index by U.S. National Oceanic and Atmospheric Administration (NOAA) on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 6.7.6
Thirsty [photo] by Dylan Alcock on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 6.7.7
Frostbitten_hands by Winky from Oxford, UK on Wikimedia Commons is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/deed.en) license.
Figure 6.7.8
- Ringed seal preparation by Ansgar Walk on Wikimedia Commons is used under a CC BY-SA 2.5 (https://creativecommons.org/licenses/by-sa/2.5/deed.en) license.
- Butchering a narwhal by Spencer & Carole on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
- Inuit children playing while the family is on seal hunt, by GRID-Arendal on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
References
It’s Okay To Be Smart. (2017, January 9). How an igloo keeps you warm. YouTube. https://www.youtube.com/watch?v=1L7EI0vKVuU&feature=youtu.be
National Geographic. (2014, April 7). Hailstones’ Inupiaq traditions | Life below zero. YouTube. https://www.youtube.com/watch?v=_Ifq73REJiM&feature=youtu.be
TED-Ed. (2014, July 21). What happens when you get heat stroke? – Douglas J. Casa. YouTube. https://www.youtube.com/watch?v=PpHM4DfPZQU&feature=youtu.be
TED-Ed. (2018, May 15). Why do we sweat? – John Murnan. YouTube. https://www.youtube.com/watch?v=fctH_1NuqCQ&feature=youtu.be
Wim Hof. (2011, June 19). Wim Hof Method. YouTube. https://www.youtube.com/watch?v=j3sY67aGFXY&feature=youtu.be

Created by: CK-12/Adapted by Christine Miller
Assembly Line
We stay alive because millions of different chemical reactions are taking place inside our bodies all the time. Each of our cells is like the busy auto assembly line pictured in Figure 3.10.1. Raw materials, half-finished products, and waste materials are constantly being used, produced, transported, and excreted. The "workers" on the cellular assembly line are mainly enzymes. These are the proteins that make biochemical reactions happen.
What Are Biochemical Reactions?
Chemical reactions that take place inside living things are called biochemical reactions. The sum of all the biochemical reactions in an organism is called metabolism. Metabolism includes both exothermic (energy-releasing) chemical reactions and endothermic (energy-absorbing) chemical reactions.
Catabolic Reactions
Exothermic reactions in organisms are called catabolic reactions. These reactions break down molecules into smaller units and release energy. An example of a catabolic reaction is the breakdown of glucose during cellular respiration, which releases energy that cells need to carry out life processes.
Anabolic Reactions
Endothermic reactions in organisms are called anabolic reactions. These reactions build up bigger molecules from smaller ones and absorb energy. An example of an anabolic reaction is the joining of amino acids to form a protein. Which type of reactions — catabolic or anabolic — do you think occur when your body digests food?
Enzymes
Most of the biochemical reactions that happen inside of living organisms require help. Why is this the case? For one thing, temperatures inside living things are usually too low for biochemical reactions to occur quickly enough to maintain life. The concentrations of reactants may also be too low for them to come together and react. Where do the biochemical reactions get the help they need to proceed? From the enzymes.
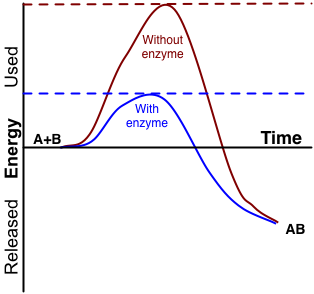
An enzyme is a protein that speeds up a biochemical reaction. It is a biological catalyst. An enzyme generally works by reducing the amount of activation energy needed to start the reaction. The graph in Figure 3.10.2 shows the activation energy needed for glucose to combine with oxygen. Less activation energy is needed when the correct enzyme is present than when it is not present.
An enzyme speeds up the reaction by lowering the required activation energy. Compare the activation energy needed with and without the enzyme.
How Well Enzymes Work
Enzymes are involved in most biochemical reactions, and they do their jobs extremely well. A typical biochemical reaction that would take several days or even several centuries to happen without an enzyme is likely to occur in just a split second with the proper enzyme! Without enzymes to speed up biochemical reactions, most organisms could not survive.
Enzymes are substrate-specific. The substrate of an enzyme is the specific substance it affects. Each enzyme works only with a particular substrate, which explains why there are so many different enzymes. In addition, for an enzyme to work, it requires specific conditions, such as the right temperature and pH. Some enzymes work best under acidic conditions, for example, while others work best in neutral environments.
Enzyme-Deficiency Disorders
There are hundreds of known inherited metabolic disorders in humans. In most of them, a single enzyme is either not produced by the body at all, or is otherwise produced in a form that doesn't work. The missing or defective enzyme is like an absentee worker on the cell's assembly line. Imagine the auto assembly line from the image at the start of this section. What if the worker who installed the steering wheel was absent? How would this impact the overall functioning of the vehicle? When an enzyme is missing, toxic chemicals build up, or an essential product isn't made. Generally, the normal enzyme is missing because the individual with the disorder inherited two copies of a gene mutation, which may have originated many generations previously.
Any given inherited metabolic disorder is generally quite rare in the general population. However, there are so many different metabolic disorders that a total of one in 1,000 to 2,500 newborns can be expected to have one.
3.10 Summary
- Biochemical reactions are chemical reactions that take place inside of living things. The sum of all of the biochemical reactions in an organism is called metabolism.
- Metabolism includes catabolic reactions, which are energy-releasing (exothermic) reactions, as well as anabolic reactions, which are energy-absorbing (endothermic) reactions.
- Most biochemical reactions need a biological catalyst called an enzyme to speed up the reaction. Enzymes reduce the amount of activation energy needed for the reaction to begin. Most enzymes are proteins that affect just one specific substance, which is called the enzyme's substrate.
- There are many inherited metabolic disorders in humans. Most of them are caused by a single defective or missing enzyme.
3.10 Review Questions
- What are biochemical reactions?
- Define metabolism.
- Compare and contrast catabolic and anabolic reactions.
- Explain the role of enzymes in biochemical reactions.
- What are enzyme-deficiency disorders?
- Explain why the relatively low temperature of living things, along with the low concentration of reactants, would cause biochemical reactions to occur very slowly in the body without enzymes.
- Answer the following questions about what happens after you eat a sandwich.
- Pieces of the sandwich go into your stomach, where there are digestive enzymes that break down the food. Which type of metabolic reaction is this? Explain your answer.
- During the process of digestion, some of the sandwich is broken down into glucose, which is then further broken down to release energy that your cells can use. Is this an exothermic endothermic reaction? Explain your answer.
- The proteins in the cheese, meat, and bread in the sandwich are broken down into their component amino acids. Then your body uses those amino acids to build new proteins. Which kind of metabolic reaction is represented by the building of these new proteins? Explain your answer.
- Explain why your body doesn’t just use one or two enzymes for all of its biochemical reactions.
- A ________ is the specific substance that an enzyme affects in a biochemical reaction.
- An enzyme is a biological _____________ .
- catabolism
- form of activation energy
- catalyst
- reactant
3.10 Explore More
https://www.youtube.com/watch?v=qgVFkRn8f10&feature=youtu.be
Enzymes (Updated), by The Amoeba Sisters, 2016.
https://www.youtube.com/watch?v=8m6RtOpqvtU&feature=youtu.be
What triggers a chemical reaction? - Kareem Jarrah, TED-Ed, 2015.
Figure 3.10.1
Auto Assembly line by Brian Snelson on Wikimedia Commons is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0) license.
Figure 3.10.2
Enzyme_activation_energy by G. Andruk [IMeowbot at the English language Wikipedia], is used under a CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0/) license.
References
Amoeba Sisters. (2016, August 28). Enzymes (updated). YouTube. https://www.youtube.com/watch?v=qgVFkRn8f10&feature=youtu.be
TED-Ed. (2015, January 15). What triggers a chemical reaction? - Kareem Jarrah. YouTube. https://www.youtube.com/watch?v=8m6RtOpqvtU&feature=youtu.be
Created by: CK-12/Adapted by Christine Miller

The Blue Marble
It's often called the "water planet," and it's been given the nickname "the blue marble." You probably just call it "home." Almost three-quarters of our home planet is covered by water, and without it, life as we know it could not exist on Earth. Water, like carbon, has a special role in living things: it is needed by all known forms of life. Although water consists of simple molecules, each containing just three atoms, its structure gives it unique properties that help explain why it is vital to all living organisms.
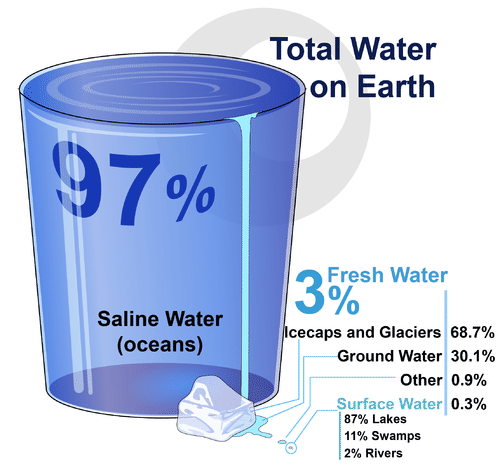
Water, Water Everywhere
If you look at Figure 3.11.2, you will see where Earth’s water is found. The term water generally refers to its liquid state, and water is a liquid over a wide range of temperatures on Earth. Water, however, also occurs on Earth as a solid (ice) and as a gas (water vapor).
Structure and Properties of Water
You are likely already aware of some of the properties of water. For example, you know that water is tasteless and odorless. You also probably know that water is transparent, which means that light can pass through it. This is important for organisms that live in the water, because some of them need sunlight to make food by photosynthesis.
Chemical Structure of Water
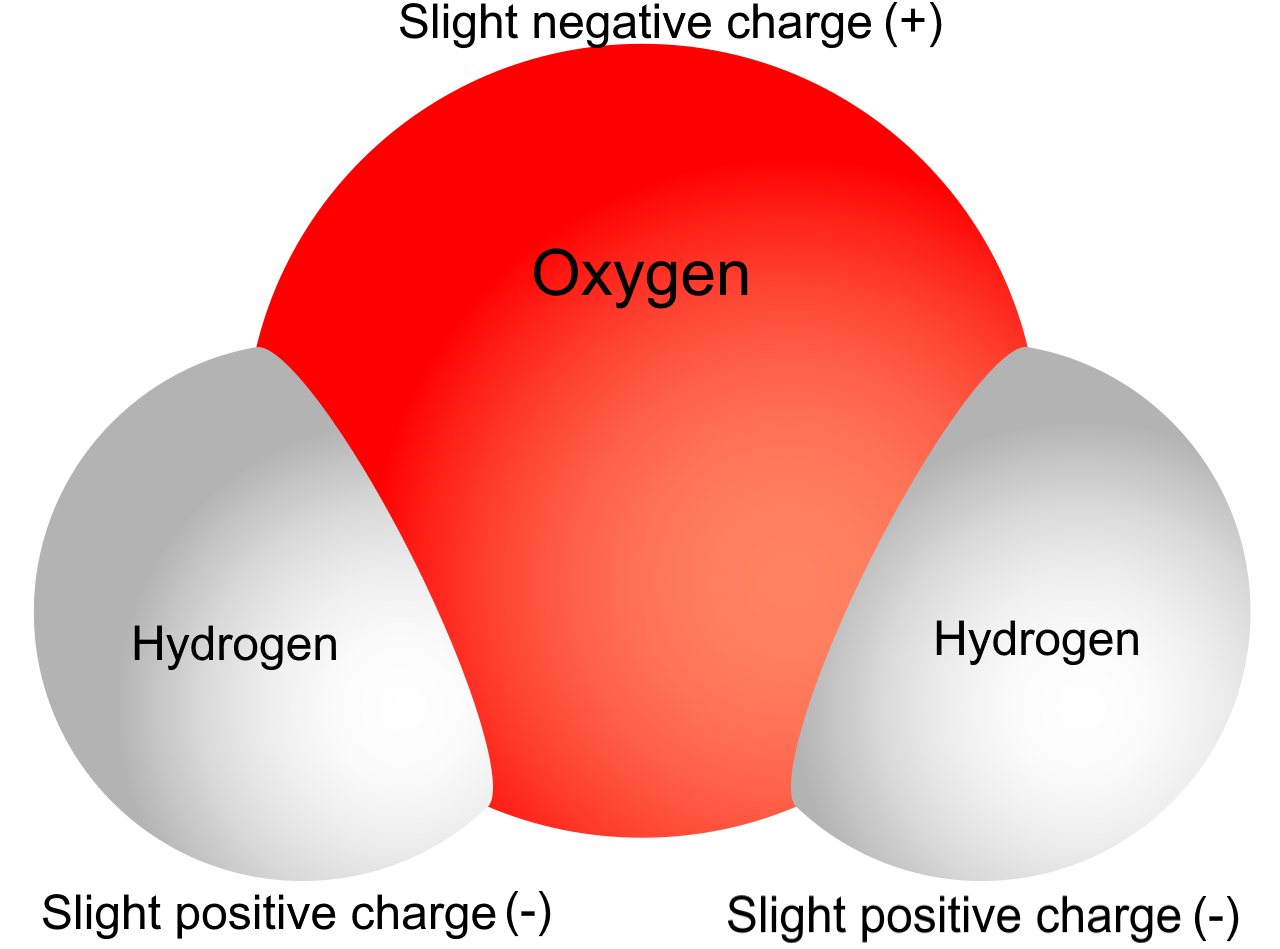
To understand some of water’s properties, you need to know more about its chemical structure. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen. The oxygen atom in a water molecule attracts electrons more strongly than the hydrogen atoms do. As a result, the oxygen atom has a slightly negative charge, and the hydrogen atoms have a slightly positive charge. A difference in electrical charge between different parts of the same molecule is called polarity. The diagram in Figure 3.11.3 shows water’s polarity.
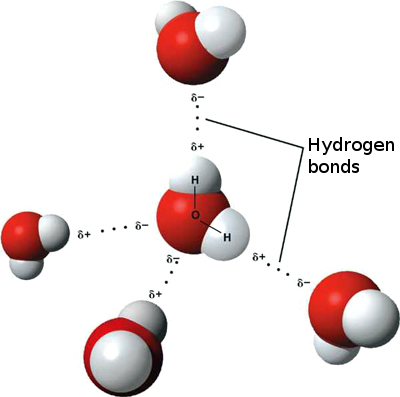
When it comes to charged molecules, opposites attract. In the case of water, the positive (hydrogen) end of one water molecule is attracted to the negative (oxygen) end of a nearby water molecule. Because of this attraction, weak bonds form between adjacent water molecules, as shown in Figure 3.11.4. The type of bond that forms between water molecules is called a hydrogen bond. Bonds between molecules are not as strong as bonds within molecules, but in water, they are strong enough to hold together nearby molecules.
How do you think hydrogen bonding affects water's properties?
Properties of Water

Hydrogen bonds between water molecules explain some of water’s properties — for example, why water molecules tend to "stick" together. Did you ever watch water drip from a leaky faucet or from a melting icicle? If you did, then you know that water always falls in drops, rather than as separate molecules. The dew drops pictured to the left are another example of water molecules sticking together.
Hydrogen bonds cause water to have a relatively high boiling point of 100°C (212°F). Extra energy is needed to break these bonds and separate water molecules so they can escape into the air as water vapor. Because of its high boiling point, most water on Earth is in a liquid state, rather than a gaseous state. Water in its liquid state is needed by all living things. Hydrogen bonds also cause water to expand when it freezes. This, in turn, causes ice to have a lower density (that is, less mass per unit volume) than liquid water. The lower density of ice means that it floats on water. In cold climates, ice floats on top of the water in lakes. This allows lake animals like fish to survive the winter by staying in the liquid water under the ice.
Watch the video below to hear more about hydrogen bonding and it's effects on the properties of water:
https://www.youtube.com/watch?v=UukRgqzk-KE
Why does ice float in water? - George Zaidan and Charles Morton, TED-ED, 2013.
Water and Living Things
The human body is about 70 per cent water (not counting the water in body fat, which varies from person to person). The body needs all this water to function normally. Just why is so much water required by human beings and other organisms? Water can dissolve many substances that organisms need. Water's polarity helps it dissolve other polar substances. Water is also necessary for many biochemical reactions. The examples below are among the most important biochemical processes that occur in living things, but they are just two of the many ways that water is involved in biochemical reactions.
- Photosynthesis: In this process, cells use the energy in sunlight to change carbon dioxide and water to glucose and oxygen. The reactions of photosynthesis can be represented by the chemical equation:
6CO2 + 6H2O + Energy → C6H12O6 + 6O2
- Cellular respiration: In this process, cells break down glucose in the presence of oxygen and release carbon dioxide, water, and energy. The reactions of cellular respiration can be represented by the chemical equation:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Water is involved in many other biochemical reactions and almost all life processes depend on water.
Feature: My Human Body

Are you a marathon runner or other endurance athlete? Do you live and work in a hot, humid climate? If you answered "yes" to either question, you may be at risk of water intoxication.
Water is considered the least toxic chemical compound, so it may surprise you to learn that drinking too much water can cause serious illness and even death. Water intoxication is a potentially fatal disturbance in brain functions. It results when the normal balance of sodium and other electrolytes in the body is pushed outside safe limits by overhydration, or taking in too much water. The condition is also called hyponatremia, which refers to a lower-than-normal level of sodium in the blood that occurs when more water is entering than leaving the body.
As excessive water is consumed, fluid outside the cells decreases in its concentration of sodium and other electrolytes relative to the concentration inside the cells. This causes fluid to enter the cells by osmosis to balance the electrolyte concentration. The extra fluid in the cells causes them to swell. In the brain, this swelling increases the pressure inside the skull. It is this increase in pressure that leads to the first observable symptoms of water intoxication, which typically include headache, confusion, irritability, and drowsiness. As the condition worsens, additional symptoms may occur, such as difficulty breathing during exertion, muscle weakness and pain, or nausea and vomiting. If the condition persists, the cells in the brain may swell to the point where blood flow is interrupted or pressure is applied to the brain stem. This is extremely dangerous and may lead to seizures, brain damage, coma, or even death.
Under normal circumstances, it is very rare to accidentally consume too much water. However, it is relatively common in athletes who participate in endurance activities, such as marathon running. A study conducted on participants of the 2002 Boston Marathon, for example, found that 13 per cent of the runners finished the race with water intoxication (Almond, et al., 2005). The study also found that water intoxication was just as likely to occur in runners who drank sports drinks containing electrolytes as those who drank plain water. Water intoxication is so common at marathon events that medical personnel who work at such events are trained to suspect water intoxication when runners collapse or show signs of confusion.
Because of the publicity water intoxication has received lately, sports experts have lowered their recommendations for water intake during endurance events. They now advise drinking only when thirsty rather than drinking to "stay ahead of thirst," which they recommended previously. Keeping water intake in line with water loss is the best way to prevent water intoxication. Mild water intoxication can be treated by restricting fluid intake. In more severe cases, treatment may require the use of diuretic drugs (which increase urination) or other types of drugs to reduce blood volume. Serious water intoxication should be considered a true medical emergency.
3.11 Summary
- Most water on Earth consists of salt water in the oceans. Only a tiny percentage of the Earth's water is fresh liquid water.
- Virtually all living things on Earth require liquid water. Water exists as a liquid over a wide range of temperatures and dissolves many substances. These properties depend on water's polarity, which causes water molecules to "stick" together.
- The human body is about 70 per cent water (outside of fat). Organisms need water to dissolve many substances and for most biochemical processes, including photosynthesis and cellular respiration.
3.11 Review Questions
- Where is most of Earth's fresh water found?
- Identify properties of water.
- What is polarity? Explain why water molecules are polar.
- Why do water molecules tend to "stick" together?
- What role does water play in photosynthesis and cellular respiration?
- Which do you think is stronger: the bonds between the hydrogen and oxygen atoms within a water molecule, or the bonds between the hydrogen and oxygen atoms between water molecules? Explain your answer.
- Given what you’ve learned about water intoxication (or hyponatremia), explain why you think drinking salt water would be bad for your cells.
- What is the name for the bonds that form between water molecules?
- Explain why water can dissolve other polar molecules.
- If there is pollution in the ocean that causes the water to become more cloudy or opaque, how do you think the ocean's photosynthetic organisms will be affected? Explain your answer.
- Describe one way in which your body gets rid of excess water.
- True or False: Ice floats on top of water because it is denser than water.
3.11 Explore More
https://www.youtube.com/watch?v=3jwAGWky98c&t=14s
Properties of Water, by The Amoeba Sisters, 2016.
https://www.youtube.com/watch?v=ASLUY2U1M-8&t=84s
How polarity makes water behave strangely - Christina Kleinberg, TED-Ed, 2013.
Attributions
Figure 3.11.1
Planet Earth by NASA (photo taken by either Harrison Schmitt or Ron Evans (of the Apollo 17 crew), on Wikimedia Commons, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 3.11.2
Total water on earth by LadyofHats at CK12, is used under a CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/) license.
Figure 3.11.3
Polarity of water by Christine Miller is released into the Public Domain (https://creativecommons.org/publicdomain/mark/1.0/).
Figure 3.11.4
Hydrogen bonds, translated by Michal Maňas (User:snek01) is released into the public domain (https://en.wikipedia.org/wiki/Public_domain). (Original uploader was Qwerter at Czech Wikipedia.)
Figure 3.11.5
Dew by Pascal Chanel on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 3.11.6
Woman in a wheelchair marathon by Kevin André on Unsplash is used under the Unsplash License (https://unsplash.com/license).
References
Almond, C.S., Shin, A.Y., Fortescue, E.B. et al. (2005, April). Hyponatremia among runners in the Boston Marathon. The New England Journal of Medicine, 352 (15), 1550–1624. doi:10.1056/NEJMoa043901. PMID 15829535.
Amoeba Sisters. (2016, July 26). Properties of Water. YouTube. https://www.youtube.com/watch?v=3jwAGWky98c&feature=youtu.be
Ruiz Villarreal, M. (LadyofHats). (2016, August 15). Figure 2. Total water on earth [digital image]. In Brainard, J., Henderson, R., CK-12's College Human Biology FlexBook® (section 3.11). CK12 Foundation. https://www.ck12.org/book/ck-12-college-human-biology/
TED-Ed. (2013, February 4). How polarity makes water behave strangely - Christina Kleinberg. YouTube. https://www.youtube.com/watch?v=ASLUY2U1M-8&feature=youtu.be
TED-Ed. (2013, October 22). Why does ice float in water? - George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=UukRgqzk-KE&feature=youtu.be

Created by: CK-12/Adapted by Christine Miller
Danger! Acid!
You probably know that batteries contain dangerous chemicals, including strong acids. Strong acids can hurt you if they come into contact with your skin or eyes. Therefore, it may surprise you to learn that your life depends on acids. There are many acids inside your body, and some of them are as strong as battery acid. Acids are needed for digestion and some forms of energy production. Genes are made of nucleic acids, proteins of amino acids, and lipids of fatty acids.
Water and Solutions
Acids (such as battery acid) are solutions. A solution is a mixture of two or more substances that has the same composition throughout. Many solutions are a mixture of water and some other substance. Not all solutions are acids. Some are bases and some are neither acids nor bases. To understand acids and bases, you need to know more about pure water.
In pure water (such as distilled water), a tiny fraction of water molecules naturally breaks down to form ions. An ion is an electrically charged atom or molecule. The breakdown of water is represented by the chemical equation:
2 H2O → H3O+ + OH-
The products of this reaction are a hydronium ion (H3O+) and a hydroxide ion (OH-). The hydroxide ion, which has a negative charge, forms when a water molecule gives up a positively charged hydrogen ion (H+). The hydronium ion, which has a positive charge, forms when another water molecule accepts the hydrogen ion.
Acidity and pH
The concentration of hydronium ions in a solution is known as acidity. In pure water, the concentration of hydronium ions is very low; only about one in ten million water molecules naturally breaks down to form a hydronium ion. As a result, pure water is essentially neutral. Acidity is measured on a scale called pH, as shown in Figure 3.12.2. Pure water has a pH of 7, so the point of neutrality on the pH scale is 7.
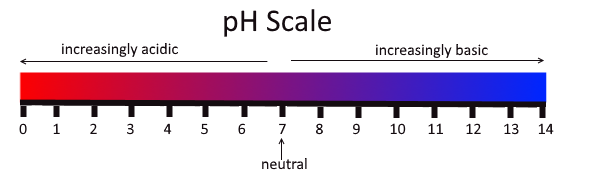
This pH scale shows the acidity of many common substances. The lower the pH value, the more acidic a substance is.

Acids
If a solution has a higher concentration of hydronium ions than pure water, it has a pH lower than 7. A solution with a pH lower than 7 is called an acid. As the hydronium ion concentration increases, the pH value decreases. Therefore, the more acidic a solution is, the lower its pH value is.
Did you ever taste vinegar? Like other acids, it tastes sour. Stronger acids can be harmful to organisms. Even stomach acid would eat through the stomach if it were not lined with a layer of mucus. Strong acids can also damage materials, even hard materials such as glass.
Bases
If a solution has a lower concentration of hydronium ions than pure water, it has a pH higher than 7. A solution with a pH higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. Like strong acids, strong bases can harm organisms and damage materials. For example, lye can burn the skin, and bleach can remove the colour from clothing.
Buffers
A buffer is a solution that can resist changes in pH. Buffers are able to maintain a certain pH by by absorbing any H+ or OH- ions added to the solution. Buffers are extremely important in biological systems in order to maintain a pH conducive to life. Bicarbonate is an example of a buffer which is used to maintain pH of the blood. In this buffering system, if blood becomes too acidic, carbonic acid will convert to carbon dioxide and water. If the blood becomes too basic, carbonic acid will convert to bicarbonate and H+ ions:
CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+
Acids, Bases, and Enzymes
Many acids and bases in living things provide the pH that enzymes need. Enzymes are biological catalysts that must work effectively for biochemical reactions to occur. Most enzymes can do their job only at a certain level of acidity. Cells secrete acids and bases to maintain the proper pH for enzymes to do their work.
Every time you digest food, acids and bases are at work in your digestive system. Consider the enzyme pepsin, which helps break down proteins in the stomach. Pepsin needs an acidic environment to do its job. The stomach secretes a strong acid called hydrochloric acid that allows pepsin to work. When stomach contents enter the small intestine, the acid must be neutralized, because enzymes in the small intestine need a basic environment in order to work. An organ called the pancreas secretes a base named bicarbonate into the small intestine, and this base neutralizes the acid.
Feature: My Human Body
Do you ever have heartburn? The answer is probably "yes." More than 60 million Americans have heartburn at least once a month, and more than 15 million suffer from it on a daily basis. Knowing more about heartburn may help you prevent it or know when it's time to seek medical treatment.
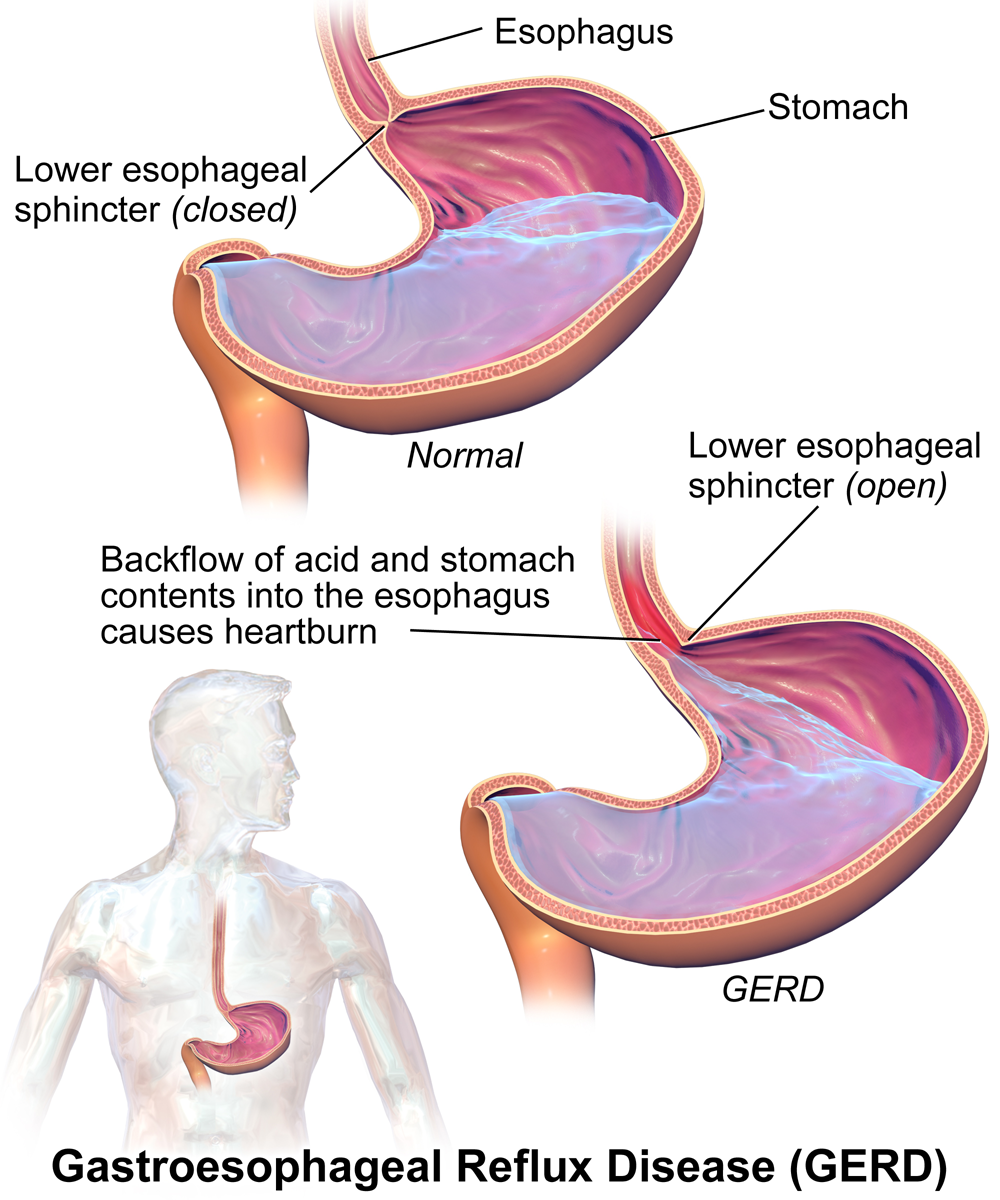
Heartburn doesn't have anything to do with the heart, but it does cause a burning sensation in the vicinity of the chest. Normally, the acid secreted into the stomach remains in the stomach where it is needed to allow pepsin to do its job of digesting proteins. A long tube called the esophagus carries food from the mouth to the stomach. A sphincter, or valve, between the esophagus and stomach opens to allow swallowed food to enter the stomach and then closes to prevent stomach contents from backflowing into the esophagus. If this sphincter is weak or relaxes inappropriately, stomach contents flow into the esophagus. Because stomach contents are usually acidic, this causes the burning sensation known as heartburn. People who are prone to heartburn and suffer from it often may be diagnosed with GERD, which stands for gastroesophageal reflux disease.
GERD — as well as occasional heartburn — often can be improved by dietary and other lifestyle changes that decrease the amount and acidity of reflux from the stomach into the esophagus.
- Some foods and beverages seem to contribute to GERD, so these should be avoided. Problematic foods include chocolate, fatty foods, peppermint, coffee, and alcoholic beverages.
- Decreasing portion size and eating the last meal of the day at least a couple of hours before bedtime may reduce the risk of reflux occurring.
- Smoking tends to weaken the lower esophageal sphincter, so quitting the habit may help control reflux.
- GERD is often associated with being overweight. Losing weight often brings improvement.
- Some people are helped by sleeping with the head of the bed elevated. This allows gravity to help control the backflow of acids into the esophagus from the stomach.
If you have frequent heartburn and lifestyle changes don't help, you may need medication to control the condition. Over-the-counter (OTC) antacids may be all that you need to control the occasional heartburn attack. OTC medications are usually bases that neutralize stomach acids. They may also create bubbles that help block stomach contents from entering the esophagus. For some people, OTC medications are not enough, and prescription medications are instead required for the control of GERD. These prescription medications generally work by inhibiting acid secretion in the stomach.
Be sure to see a doctor if you can't control your heartburn, or you have it often. Untreated GERD not only interferes with quality of life, it may also lead to more serious complications, ranging from esophageal bleeding to esophageal cancer.
3.12 Summary
- A solution is a mixture of two or more substances that has the same composition throughout. Many solutions consist of water and one or more dissolved substances.
- Acidity is a measure of the hydronium ion concentration in a solution. Pure water has a very low concentration and a pH of 7, which is the point of neutrality on the pH scale.
- Acids have a higher hydronium ion concentration than pure water and a pH lower than 7. Bases have a lower hydronium ion concentration than pure water and a pH higher than 7.
- Many acids and bases in living things are secreted to provide the proper pH for enzymes to work properly. Enzymes are the biological catalysts (like pepsin) needed to digest protein in the stomach. Pepsin requires an acidic environment.
3.12 Review Questions
-
- What is a solution?
- Define acidity.
- Explain how acidity is measured.
- Compare and contrast acids and bases.
- Hydrochloric acid is secreted by the stomach to provide an acidic environment for the enzyme pepsin. What is the pH of this acid? How strong of an acid is it compared with other acids?
- Define an ion. Identify the ions in the equation below, and explain what makes them ions:
- 2 H2O → H3O+ + OH-
- Explain why the pancreas secretes bicarbonate into the small intestine.
- Do you think pepsin would work in the small intestine? Why or why not?
- You may have mixed vinegar and baking soda and noticed that they bubble and react with each other. Explain why this happens. Explain also what happens to the pH of this solution after you mix the vinegar and baking soda.
- Pregnancy hormones can cause the lower esophageal sphincter to relax. What effect do you think this has on pregnant women? Explain your answer.
3.12 Explore More
https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
pH and Buffers by Bozeman Science, 2014.
https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton, TED-Ed, 2013.
Attributions
Figure 3.12.1
Leaky battery by Carbon Arc on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
Figure 3.12.2
PH_Scale by Christinelmiller on Wikimedia Commons is used under a © CC0 1.0 (https://creativecommons.org/publicdomain/zero/1.0/) public domain dedication license.
Figure 3.12.3
Ph scale with examples by OpenStax College, on Wikimedia Commons, is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 3.12.4
GERD by BruceBlaus on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
References
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 26.15 The pH Scale [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/26-4-acid-base-balance
Bozeman Science. (2014, February 22). pH and buffers. YouTube. https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
TED-Ed. (2013, October 24). The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
Created by: CK-12/Adapted by Christine Miller


A Bag Full of Jell-O
The simple cut-away model of an animal cell (Figure 4.4.1) shows that a cell resembles a plastic bag full of Jell-O. Its basic structure is a plasma membrane filled with cytoplasm. Like Jell-O containing mixed fruit (Figure 4.4.2), the cytoplasm of the cell also contains various structures, including a nucleus and other organelles. Your body is composed of trillions of cells, but all of them perform the same basic life functions. They all obtain and use energy, respond to the environment, and reproduce. How do your cells carry out these basic functions and keep themselves — and you — alive? To answer these questions, you need to know more about the structures that make up cells, starting with the plasma membrane.
What is the Plasma Membrane?
The plasma membrane is a structure that forms a barrier between the cytoplasm inside the cell and the environment outside the cell. Without the plasma membrane, there would be no cell. Although it is very thin and flexible, the plasma membrane protects and supports the cell by controlling everything that enters and leaves it. It allows only certain substances to pass through, while keeping others in or out. To understand how the plasma membrane controls what passes into or out of the cell, you need to know its basic structure.
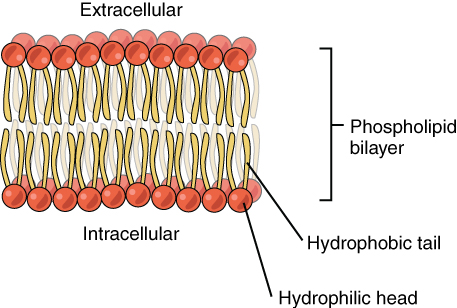
Phospholipid Bilayer
The plasma membrane is composed mainly of phospholipids, which consist of fatty acids and alcohol. The phospholipids in the plasma membrane are arranged in two layers, called a phospholipid bilayer. As shown in the simplified diagram in Figure 4.4.3, each individual phospholipid molecule has a phosphate group head (in red) and two fatty acid tails (in yellow). The head “loves” water (hydrophilic) and the tails “hate” water (hydrophobic). The water-hating tails are on the interior of the membrane, whereas the water-loving heads point outward, toward either the cytoplasm (intracellular) or the fluid that surrounds the cell (extracellular).
Hydrophobic molecules can easily pass through the plasma membrane if they are small enough, because they are water-hating like the interior of the membrane. Hydrophilic molecules, on the other hand, cannot pass through the plasma membrane — at least not without help — because they are water-loving like the exterior of the membrane.
Other Molecules in the Plasma Membrane
The plasma membrane also contains other molecules, primarily other lipids and proteins. The yellow molecules in the diagram here, for example, are the lipid cholesterol. Molecules of the steroid lipid cholesterol help the plasma membrane keep its shape. Proteins in the plasma membrane (shown blue in Figure 4.4.4) include: transport proteins that assist other substances in crossing the cell membrane, receptors that allow the cell to respond to chemical signals in its environment, and cell-identity markers that indicate what type of cell it is and whether it belongs in the body.
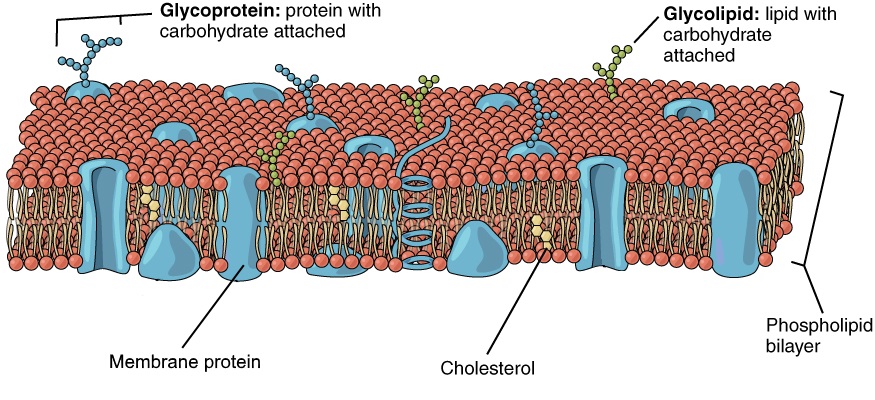
Additional Functions of the Plasma Membrane
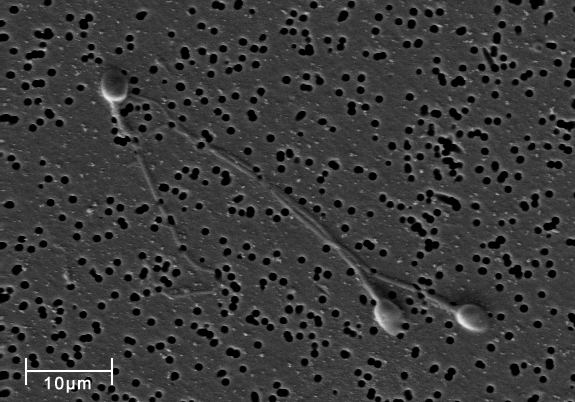
The plasma membrane may have extensions, such as whip-like flagella (singular flagellum) or brush-like cilia (singular cilium), shown below (Figure 4.4.5), that give it other functions. In single-celled organisms, these membrane extensions may help the organisms move. In multicellular organisms, the extensions have different functions. For example, the cilia on human lung cells sweep foreign particles and mucus toward the mouth and nose, while the flagellum on a human sperm cell allows it to swim.

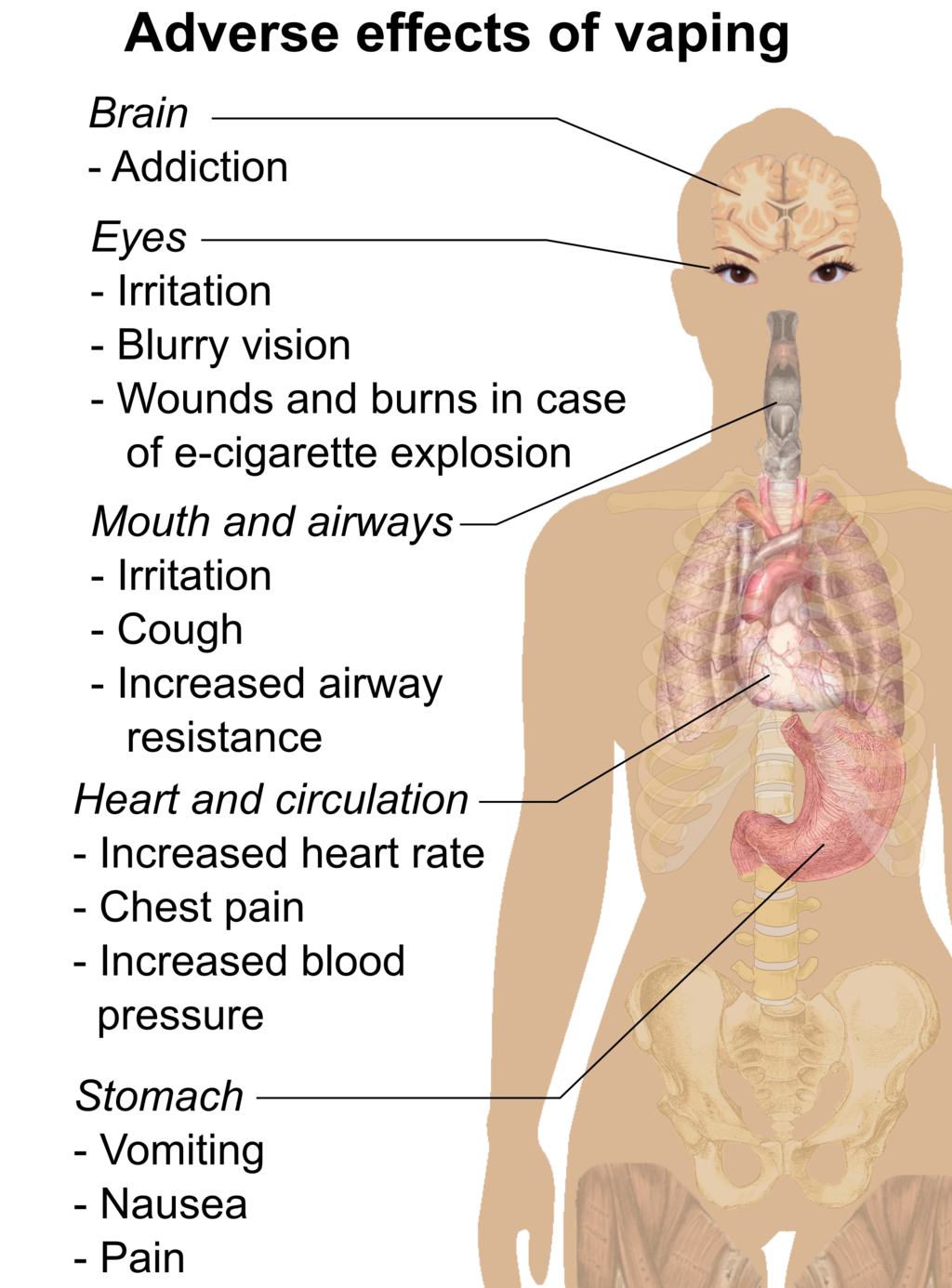
Feature: My Human Body
If you smoke or use e-cigarettes (vaping) and need another reason to quit, here's a good one. We usually think of lung cancer as the major disease caused by smoking. But smoking and vaping can have devastating effects on the body's ability to protect itself from repeated, serious respiratory infections, such as bronchitis and pneumonia.
Cilia are microscopic, hair-like projects on cells that line the respiratory, reproductive, and digestive systems. Cilia in the respiratory system line most of your airways, where they have the job of trapping and removing dust, germs, and other foreign particles before they can make you sick. Cilia secrete mucus that traps particles, and they move in a continuous wave-like motion that sweeps the mucus and particles upward toward the throat, where they can be expelled from the body. When you are sick and cough up phlegm, that's what you are doing.
Smoking prevents cilia from performing these important functions. Chemicals in tobacco smoke paralyze the cilia so they can't sweep mucus out of the airways. Those chemicals also inhibit the cilia from producing mucus. Fortunately, these effects start to wear off soon after the most recent exposure to tobacco smoke. If you stop smoking, your cilia will return to normal. Even if prolonged smoking has destroyed cilia, they will regrow and resume functioning in a matter of months after you stop smoking.
4.4 Summary
- The plasma membrane is a structure that forms a barrier between the cytoplasm inside the cell and the environment outside the cell. It allows only certain substances to pass in or out of the cell.
- The plasma membrane is composed mainly of a bilayer of phospholipid molecules. It also contains other molecules, such as the steroid cholesterol, which helps the membrane keep its shape, and transport proteins, which help substances pass through the membrane.
- The plasma membranes of some cells have extensions that have other functions, like flagella to help sperm move, or cilia to help keep our airways clear.
4.4 Review Questions
- What are the general functions of the plasma membrane?
- Describe the phospholipid bilayer of the plasma membrane.
- Identify other molecules in the plasma membrane. State their functions.
- Why do some cells have plasma membrane extensions, like flagella and cilia?
- Explain why hydrophilic molecules cannot easily pass through the cell membrane. What type of molecule in the cell membrane might help hydrophilic molecules pass through it?
- Which part of a phospholipid molecule in the plasma membrane is made of fatty acid chains? Is this part hydrophobic or hydrophilic?
- The two layers of phospholipids in the plasma membrane are called a phospholipid ____________.
-
- Steroid hormones can pass directly through cell membranes. Why do you think this is the case?
- Some antibiotics work by making holes in the plasma membrane of bacterial cells. How do you think this kills the cells?
- What is the name of the long, whip-like extensions of the plasma membrane that helps some single-celled organisms move?
4.4 Explore More
https://www.youtube.com/watch?v=yAXnYcUjn5k&feature=emb_logo
Insights into cell membranes via dish detergent - Ethan Perlstein, TED-Ed, 2013.
https://www.youtube.com/watch?v=qBCVVszQQNs
Inside the cell membrane, by The Amoeba Sisters, 2018.
Attributions
Figure 4.4.1
Animal Cell Unannotated, by Kelvin Song on Wikimedia Commons is used under a CC0 1.0 (https://creativecommons.org/publicdomain/zero/1.0/deed.en) public domain dedication license.
Figure 4.4.2
Jello mold at the mexican bakery photo by Aimée Knight on Flickr is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/) license.
Figure 4.4.3
Phospholipid_Bilayer by OpenStax on Wikimedia Commons is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
Figure 4.4.4
Lipid bilayer by OpenStax on Wikimedia Commons is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
Figure 4.4.5
Spermatozoa-human-3140x by No specific author on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.4.6
Cilia/ Bronchiolar epithelium 3 - SEM by Charles Daghlian on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.4.7
Adverse effects of vaping (raster) by Mikael Häggström on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
References
Amoeba Sisters. (2018, February 27). Inside the cell membrane. YouTube. https://www.youtube.com/watch?v=qBCVVszQQNs&feature=youtu.be
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E.. Womble, M., DeSaix. P. (2013, April 25). Figure 3.3 Phospolipid Bilayer [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/3-1-the-cell-membrane
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E.. Womble, M., DeSaix. P. (2013, April 25). Figure 3.4 Cell Membrane [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/3-1-the-cell-membrane
Ghosh, A., Coakley, R. C., Mascenik, T., Rowell, T. R., Davis, E. S., Rogers, K., Webster, M. J., Dang, H., Herring, L. E., Sassano, M. F., Livraghi-Butrico, A., Van Buren, S. K., Graves, L. M., Herman, M. A., Randell, S. H., Alexis, N. E., & Tarran, R. (n.d.). Chronic E-Cigarette Exposure Alters the Human Bronchial Epithelial Proteome. American Journal of Respiratory and Critical /Care Medicine, 198(1), 67–76. https://doi-org.ezproxy.tru.ca/10.1164/rccm.201710-2033OC
TED-Ed. (2013, February 26). Insights into cell membranes via dish detergent - Ethan Perlstein. YouTube. https://www.youtube.com/watch?v=yAXnYcUjn5k&feature=youtu.be
The principle holding that in a warm-blooded animal species having distinct geographic populations, the limbs, ears, and other appendages of the animals living in cold climates tend to be shorter than in animals of the same species living in warm climates.
Created by: CK-12/Adapted by Christine Miller

Bring on the S'mores!
This inviting camp fire can be used for both heat and light. Heat and light are two forms of energy that are released when a fuel like wood is burned. The cells of living things also get energy by "burning." They "burn" glucose in a process called cellular respiration.
What Is Cellular Respiration?
Cellular respiration is the process by which living cells break down glucose molecules and release energy. The process is similar to burning, although it doesn’t produce light or intense heat as a campfire does. This is because cellular respiration releases the energy in glucose slowly and in many small steps. It uses the energy released to form molecules of ATP, the energy-carrying molecules that cells use to power biochemical processes. In this way, cellular respiration is an example of energy coupling: glucose is broken down in an exothermic reaction, and then the energy from this reaction powers the endothermic reaction of the formation of ATP. Cellular respiration involves many chemical reactions, but they can all be summed up with this chemical equation:
C6H12O6 6O2 → 6CO2 6H2O Chemical Energy (in ATP)
In words, the equation shows that glucose (C6H12O6) and oxygen (O2) react to form carbon dioxide (CO2) and water (H2O), releasing energy in the process. Because oxygen is required for cellular respiration, it is an aerobic process.
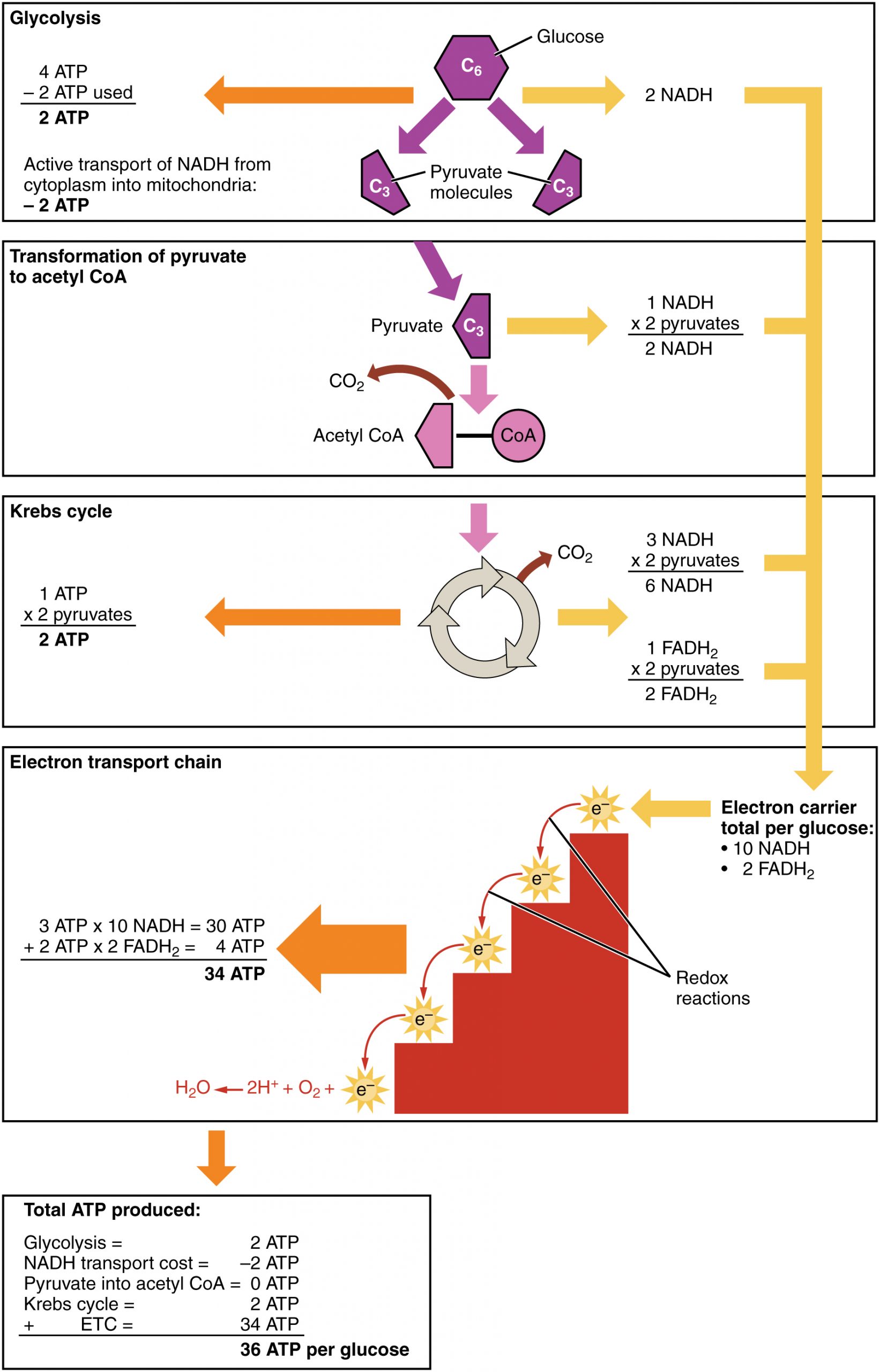
Cellular respiration occurs in the cells of all living things, both autotrophs and heterotrophs. All of them burn glucose to form ATP. The reactions of cellular respiration can be grouped into three stages: glycolysis, the Krebs cycle (also called the citric acid cycle), and electron transport. Figure 4.10.2 gives an overview of these three stages, which are also described in detail below.
Cellular Respiration Stage I: Glycolysis
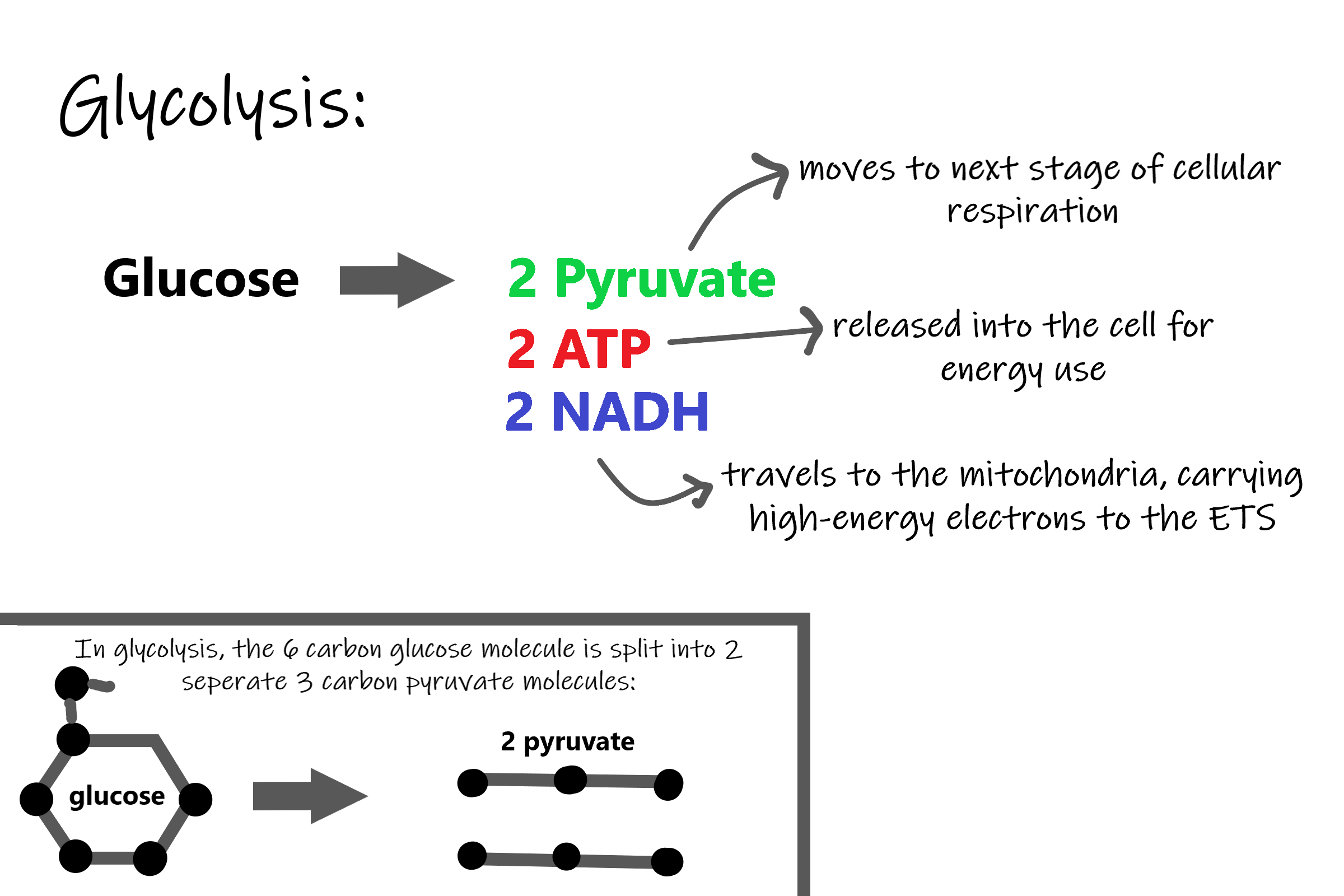
The first stage of cellular respiration is glycolysis, which happens in the cytosol of the cytoplasm.
Splitting Glucose
The word glycolysis literally means “glucose splitting,” which is exactly what happens in this stage. Enzymes split a molecule of glucose into two molecules of pyruvate (also known as pyruvic acid). This occurs in several steps, as summarized in the following diagram.
Results of Glycolysis
Energy is needed at the start of glycolysis to split the glucose molecule into two pyruvate molecules which go on to stage II of cellular respiration. The energy needed to split glucose is provided by two molecules of ATP; this is called the energy investment phase. As glycolysis proceeds, energy is released, and the energy is used to make four molecules of ATP; this is the energy harvesting phase. As a result, there is a net gain of two ATP molecules during glycolysis. During this stage, high-energy electrons are also transferred to molecules of NAD to produce two molecules of NADH, another energy-carrying molecule. NADH is used in stage III of cellular respiration to make more ATP.
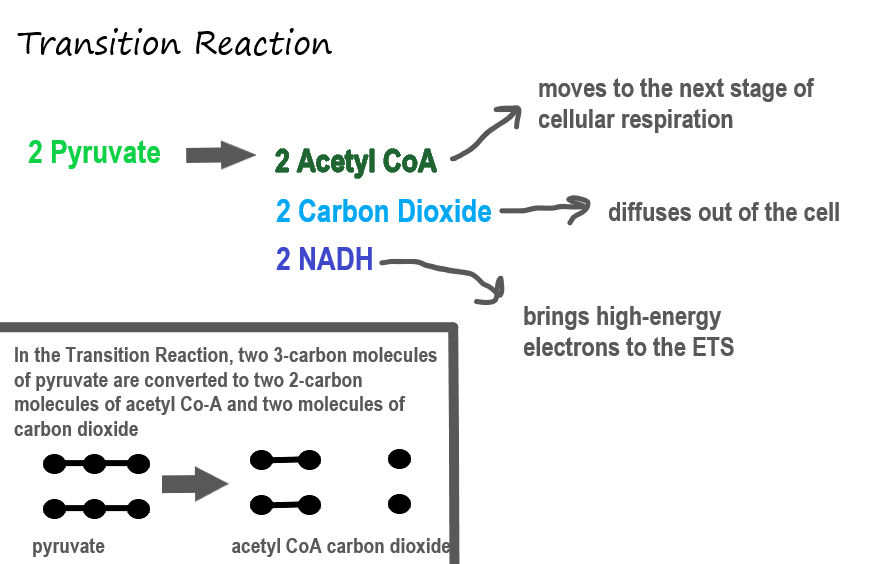
Transition Reaction
Before pyruvate can enter the next stage of cellular respiration it needs to be modified slightly. The transition reaction is a very short reaction which converts the two molecules of pyruvate to two molecules of acetyl CoA, carbon dioxide, and two high energy electron pairs convert NAD to NADH. The carbon dioxide is released, the acetyl CoA moves to the mitochondria to enter the Kreb's Cycle (stage II), and the NADH carries the high energy electrons to the Electron Transport System (stage III).
Structure of the Mitochondrion
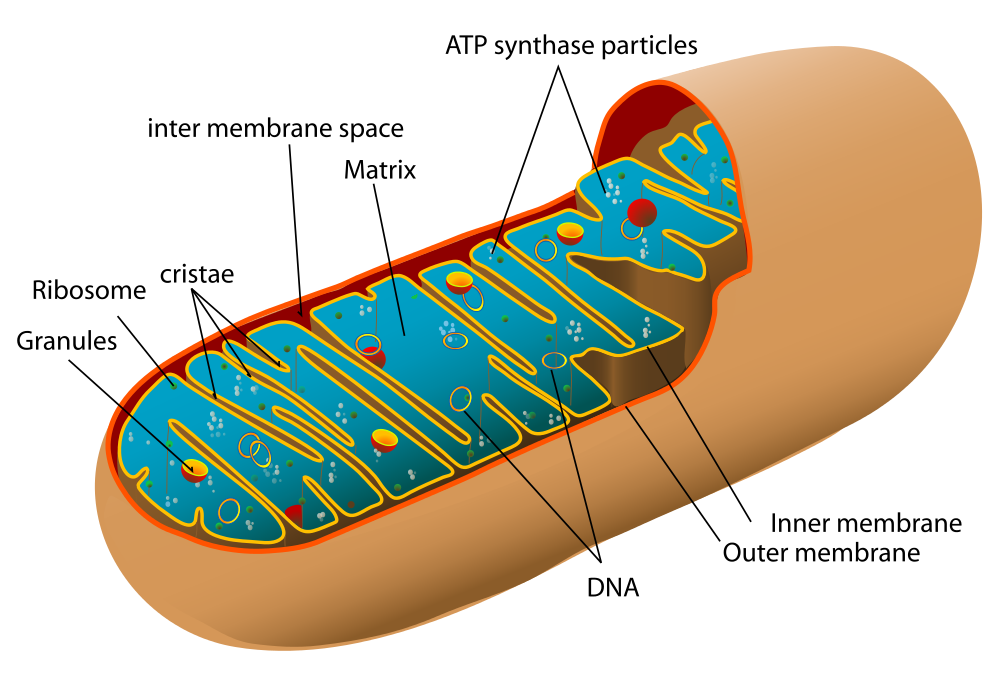
Before you read about the last two stages of cellular respiration, you need to know more about the mitochondrion, where these two stages take place. A diagram of a mitochondrion is shown in Figure 4.10.5.
The structure of a mitochondrion is defined by an inner and outer membrane. This structure plays an important role in aerobic respiration.
As you can see from the figure, a mitochondrion has an inner and outer membrane. The space between the inner and outer membrane is called the intermembrane space. The space enclosed by the inner membrane is called the matrix. The second stage of cellular respiration (the Krebs cycle) takes place in the matrix. The third stage (electron transport) happens on the inner membrane.
Cellular Respiration Stage II: The Krebs Cycle
Recall that glycolysis produces two molecules of pyruvate (pyruvic acid), which are then converted to acetyl CoA during the short transition reaction. These molecules enter the matrix of a mitochondrion, where they start the Krebs cycle (also known as the Citric Acid Cycle). The reason this stage is considered a cycle is because a molecule called oxaloacetate is present at both the beginning and end of this reaction and is used to break down the two molecules of acetyl CoA. The reactions that occur next are shown in Figure 4.10.6.
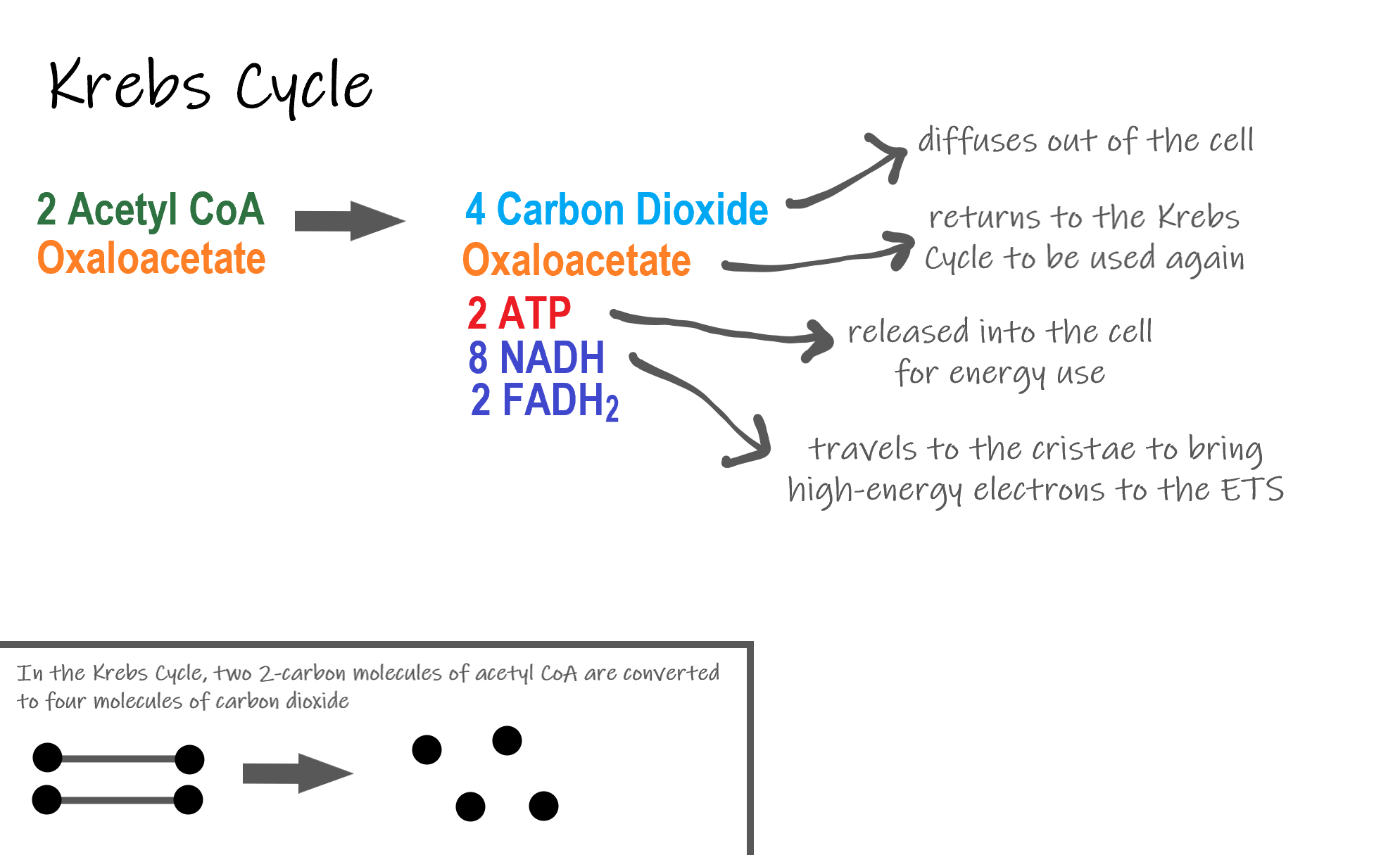
Steps of the Krebs Cycle
The Krebs cycle itself actually begins when acetyl-CoA combines with a four-carbon molecule called OAA (oxaloacetate) (see Figure 4.10.6). This produces citric acid, which has six carbon atoms. This is why the Krebs cycle is also called the citric acid cycle.
After citric acid forms, it goes through a series of reactions that release energy. The energy is captured in molecules of NADH, ATP, and FADH2, another energy-carrying coenzyme. Carbon dioxide is also released as a waste product of these reactions.
The final step of the Krebs cycle regenerates OAA, the molecule that began the Krebs cycle. This molecule is needed for the next turn through the cycle. Two turns are needed because glycolysis produces two pyruvic acid molecules when it splits glucose.
Results of the Glycolysis, Transition Reaction and Krebs Cycle
After glycolysis, transition reaction, and the Krebs cycle, the glucose molecule has been broken down completely. All six of its carbon atoms have combined with oxygen to form carbon dioxide. The energy from its chemical bonds has been stored in a total of 16 energy-carrier molecules. These molecules are:
- 4 ATP (2 from glycolysis, 2 from Krebs Cycle)
- 12 NADH (2 from glycolysis, 2 from transition reaction, and 8 from Krebs cycle)
- 2 FADH2 (both from the Krebs cycle)
The events of cellular respiration up to this point are exergonic reactions- they are releasing energy that had been stored in the bonds of the glucose molecule. This energy will be transferred to the third and final stage of cellular respiration: the Electron Transport System, which is an endergonic reaction. Using an exothermic reaction to power an endothermic reaction is known as energy coupling.
Cellular Respiration Stage III: Electron Transport Chain
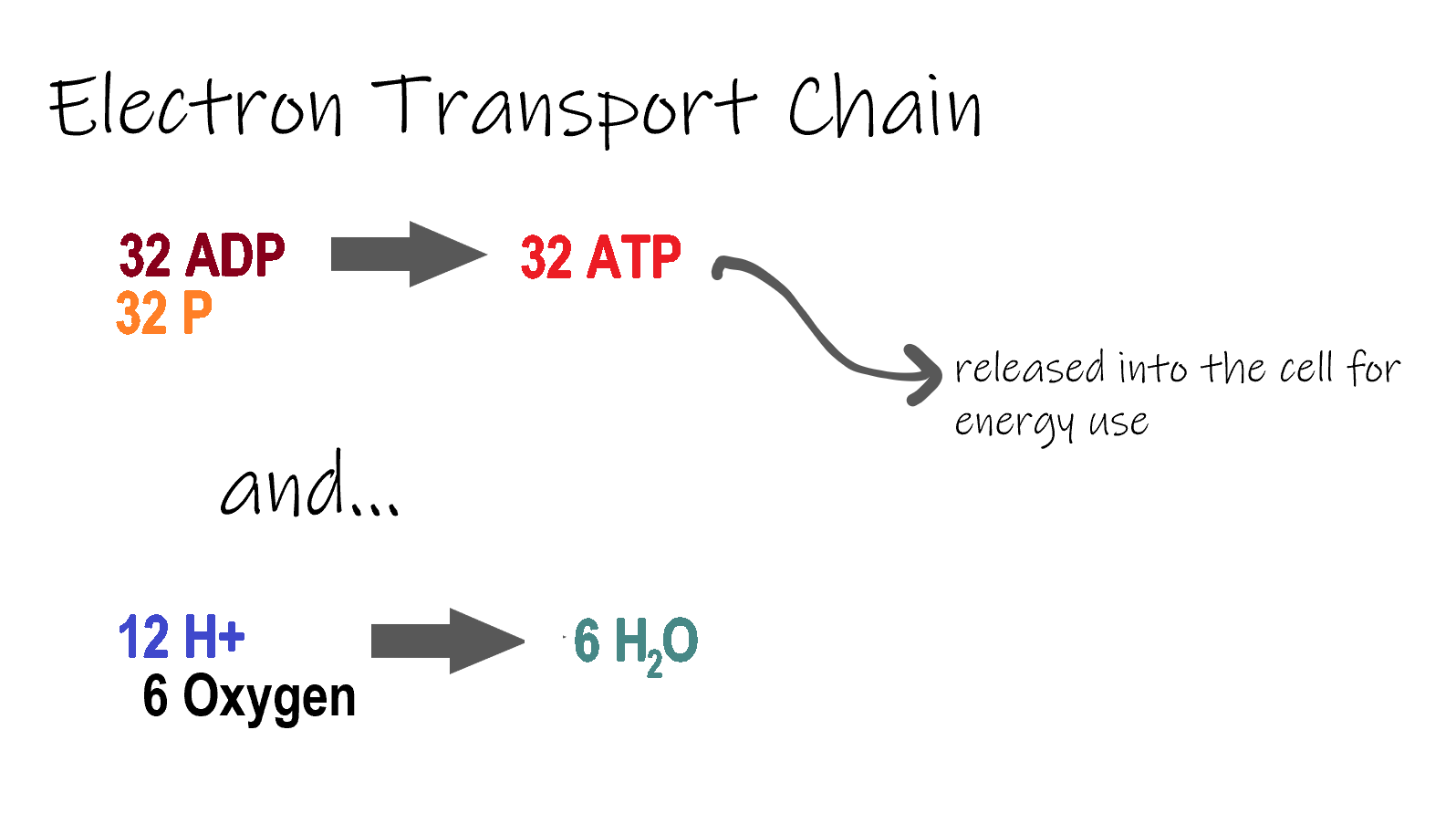
ETC, the final stage in cellular respiration produces 32 ATP. The Electron Transport Chain is the final stage of cellular respiration. In this stage, energy being transported by NADH and FADH2 is transferred to ATP. In addition, oxygen acts as the final proton acceptor for the hydrogens released from all the NADH and FADH2, forming water. Figure 4.10.8 shows the reactants and products of the ETC.
Transporting Electrons
The Electron transport chain is the third stage of cellular respiration and is illustrated in Figure 4.10.8. During this stage, high-energy electrons are released from NADH and FADH2, and they move along electron-transport chains on the inner membrane of the mitochondrion. An electron-transport chain is a series of molecules that transfer electrons from molecule to molecule by chemical reactions. Some of the energy from the electrons is used to pump hydrogen ions (H ) across the inner membrane, from the matrix into the intermembrane space. This ion transfer creates an electrochemical gradient that drives the synthesis of ATP.
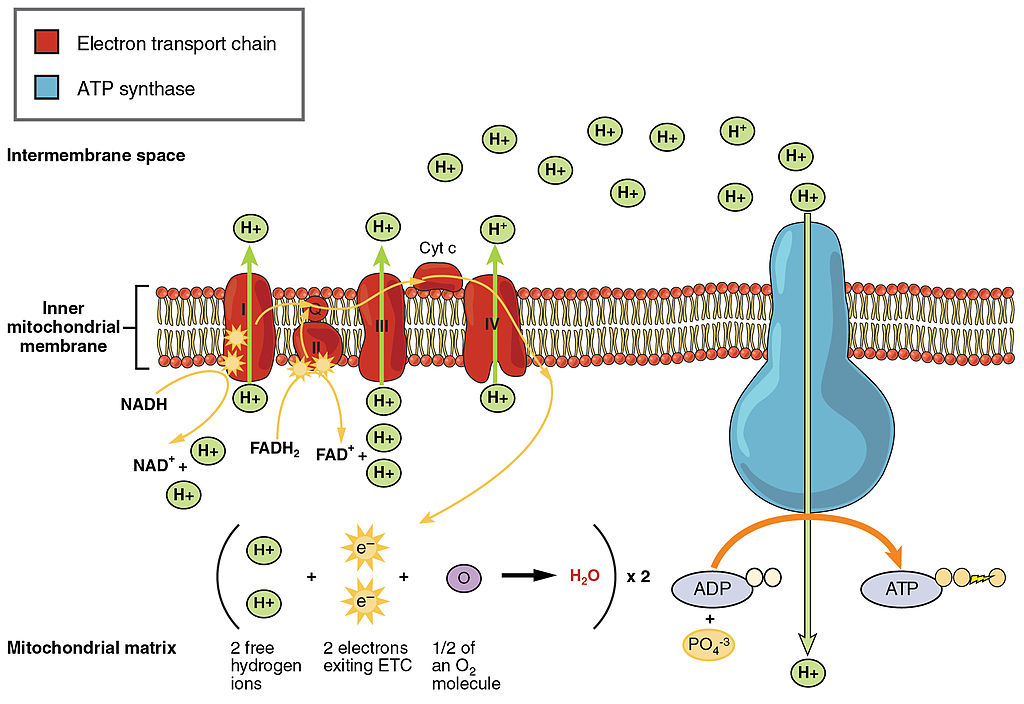
Making ATP
As shown in Figure 4.10.8, the pumping of hydrogen ions across the inner membrane creates a greater concentration of the ions in the intermembrane space than in the matrix. This gradient causes the ions to flow back across the membrane into the matrix, where their concentration is lower. ATP synthase acts as a channel protein, helping the hydrogen ions cross the membrane. It also acts as an enzyme, forming ATP from ADP and inorganic phosphate in a process called oxidative phosphorylation. After passing through the electron-transport chain, the “spent” electrons combine with oxygen to form water.
How Much ATP?
You have seen how the three stages of aerobic respiration use the energy in glucose to make ATP. How much ATP is produced in all three stages combined? Glycolysis produces two ATP molecules, and the Krebs cycle produces two more. Electron transport begins with several molecules of NADH and FADH2 from the Krebs cycle and transfers their energy into as many as 34 more ATP molecules. All told, then, up to 38 molecules of ATP can be produced from just one molecule of glucose in the process of cellular respiration.
4.10 Summary
- Cellular respiration is the aerobic process by which living cells break down glucose molecules, release energy, and form molecules of ATP. Generally speaking, this three-stage process involves glucose and oxygen reacting to form carbon dioxide and water.
- The first stage of cellular respiration, called glycolysis, takes place in the cytoplasm. In this step, enzymes split a molecule of glucose into two molecules of pyruvate, which releases energy that is transferred to ATP. Following glycolysis, a short reaction called the transition reaction converts the pyruvate into two molecules of acetyl CoA.
- The organelle called a mitochondrion is the site of the other two stages of cellular respiration. The mitochondrion has an inner and outer membrane separated by an intermembrane space, and the inner membrane encloses a space called the matrix.
- The second stage of cellular respiration, called the Krebs cycle, takes place in the matrix of a mitochondrion. During this stage, two turns through the cycle result in all of the carbon atoms from the two pyruvate molecules forming carbon dioxide and the energy from their chemical bonds being stored in a total of 16 energy-carrying molecules (including two from glycolysis and two from transition reaction).
- The third and final stage of cellular respiration, called electron transport, takes place on the inner membrane of the mitochondrion. Electrons are transported from molecule to molecule down an electron-transport chain. Some of the energy from the electrons is used to pump hydrogen ions across the membrane, creating an electrochemical gradient that drives the synthesis of many more molecules of ATP.
- In all three stages of cellular respiration combined, as many as 38 molecules of ATP are produced from just one molecule of glucose.
4.10 Review Questions
- What is the purpose of cellular respiration? Provide a concise summary of the process.
- State what happens during glycolysis.
- Describe the structure of a mitochondrion.
- What molecule is present at both the beginning and end of the Krebs cycle?
- What happens during the electron transport stage of cellular respiration?
- How many molecules of ATP can be produced from one molecule of glucose during all three stages of cellular respiration combined?
- Do plants undergo cellular respiration? Why or why not?
- Explain why the process of cellular respiration described in this section is considered aerobic.
- Name three energy-carrying molecules involved in cellular respiration.
-
- Which stage of aerobic cellular respiration produces the most ATP?
-
4.10 Explore More
https://www.youtube.com/watch?time_continue=2&v=00jbG_cfGuQ&feature=emb_logo
ATP & Respiration: Crash Course Biology #7, CrashCourse, 2012.
https://www.youtube.com/watch?v=4Eo7JtRA7lg&t=3s
Cellular Respiration and the Mighty Mitochondria, The Amoeba Sisters, 2014.
Attributions
Figure 4.10.1
Smores by Jessica Ruscello on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 4.10.2
Carbohydrate_Metabolism by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 4.10.3
Glycolysis by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.4
Transition Reaction by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.5
Mitochondrion by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.10.6
Krebs cycle by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.7
Electron Transport Chain (ETC) by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.8
The_Electron_Transport_Chain by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
References
CrashCourse. (2012, March 12). ATP & Respiration: Crash Course Biology #7. YouTube. https://www.youtube.com/watch?time_continue=2&v=00jbG_cfGuQ&feature=emb_logo
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 24.8 Electron Transport Chain [digital image]. In Anatomy & Physiology, Connexions (Section ). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/24-2-carbohydrate-metabolism
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 24.9 Carbohydrate Metabolism [digital image]. In Anatomy & Physiology, Connexions (Section 24.2). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/24-2-carbohydrate-metabolism
The Amoeba Sisters. (2014, October 22). Cellular Respiration and the Mighty Mitochondria. YouTube. https://www.youtube.com/watch?v=4Eo7JtRA7lg&t=3s
The transfer of genetic variation from one population to another. If the rate of gene flow is high enough, then two populations are considered to have equivalent allele frequencies and therefore effectively be a single population.
A thick band of nerve fibers that divides the cerebral cortex lobes into left and right hemispheres. It connects the left and right sides of the brain, allowing for communication between both hemispheres.
Any steroid hormone produced by the cortex of the adrenal gland; includes mineralocorticoids, glucocorticoids, and androgens.
The number of calories required to keep your body functioning at rest.

