3.9 Energy in Chemical Reactions
Slow Burn

This old truck gives off a small amount of heat as it rusts. The rusting of iron is a chemical process. It occurs when iron and oxygen go through a chemical reaction similar to burning, or combustion. Obviously, the chemical reaction that occurs when something burns gives off energy. You can feel the heat, and you may be able to see the light of flames. The rusting of iron is a much slower process, but it still gives off energy. It’s just that it releases energy so slowly that you can’t detect a change in temperature.
The Role of Energy in Chemical Reactions
Matter rusting or burning are common examples of chemical changes. Chemical changes involve chemical reactions, in which some substances, called reactants, change at the molecular level to form new substances, which are called products. All chemical reactions involve energy, but not all chemical reactions release energy, like rusting and burning. In some chemical reactions, energy is absorbed rather than released.
Exothermic Reactions

A chemical reaction that releases energy is called an exothermic reaction. This type of reaction can be represented with this general chemical equation:
Reactants → Products + Heat
Another example of an exothermic reaction is chlorine combining with sodium to form table salt. The decomposition of organic matter also releases energy because of exothermic reactions. Sometimes on a chilly morning, you can see steam rising from a compost pile because of these chemical reactions (see photo in Figure 3.9.2).
This compost pile is steaming because it is much warmer than the chilly air around it. The heat comes from all the exothermic chemical reactions taking place inside the compost as it decomposes.
A special type of exothermic reaction is an exergonic reaction– not only do exergonic reactions release energy, but in addition, they occur spontaneously. Many cell processes rely on exergonic reactions: in a chemical process called cellular respiration, which is similar to combustion, the sugar glucose is “burned” to provide cells with energy.
Endothermic Reactions
A chemical reaction that absorbs energy is called an endothermic reaction. This type of reaction can also be represented by a general chemical equation:
Reactants + Energy → Products
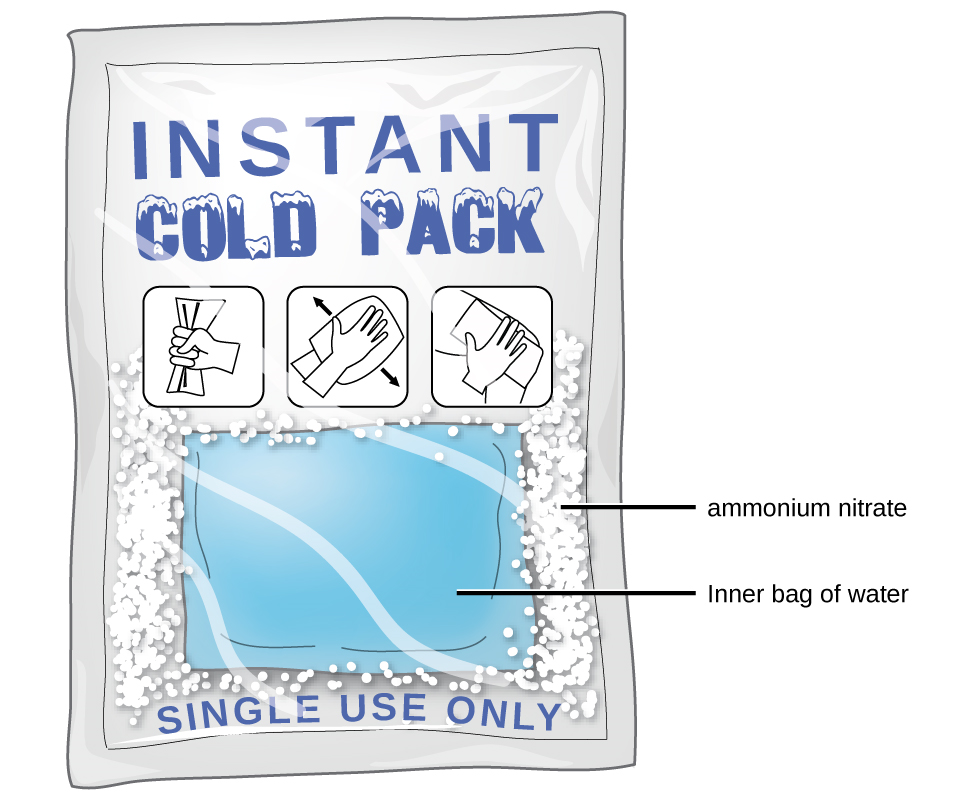
Did you ever use a chemical cold pack like the one pictured? The pack cools down because of an endothermic reaction. When a tube inside the pack is broken, it releases ammonium nitrate, a chemical that reacts with water inside the pack. This reaction absorbs heat energy and quickly cools down the contents of the pack.
Many other chemical processes involve endothermic reactions. Most cooking and baking, for example, involves the use of energy to produce chemical reactions. You can’t bake a cake or cook an egg without adding heat energy.
Arguably, the most important endothermic reactions occur during photosynthesis. When plants produce sugar by photosynthesis, they take in light energy to power the necessary endothermic reactions. The sugar they produce provides plants and virtually all other living things with glucose for cellular respiration.
Activation Energy
All chemical reactions require energy to get started. Even reactions that release energy need a boost of energy in order to begin. The energy needed to start a chemical reaction is called activation energy. Activation energy is like the push a child needs to start going down a playground slide. The push gives the child enough energy to start moving, but once she starts, she keeps moving without being pushed again. Activation energy is illustrated in the graph in Figure 3.9.4.
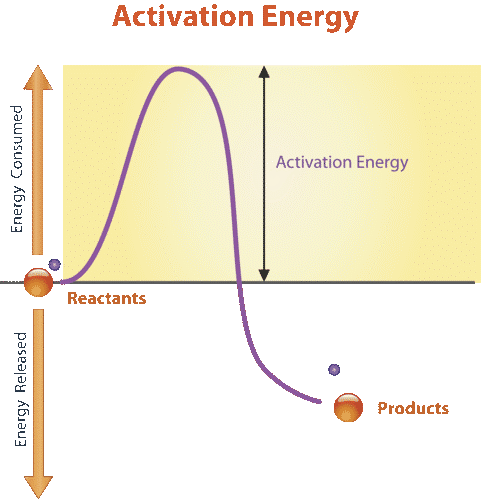
Why do chemical reactions need energy to get started? In order for reactions to begin, reactant molecules must bump into each other, so they must be moving — and movement requires energy. When reactant molecules bump together, they may repel each other because of intermolecular forces pushing them apart. Energy is also required to overcome these forces so the molecules can come together and react.
3.9 Summary
- All chemical reactions involve energy. Exothermic reactions release energy. Endothermic reactions absorb energy.
- All chemical reactions need activation energy to begin. Activation energy provides the “push” needed to get the reaction started.
3.9 Review Questions
- Compare endothermic and exothermic chemical reactions. Give an example of a process that involves each type of reaction.
- Define activation energy.
- Explain why chemical reactions require activation energy.
- Heat is a form of ____________ .
- In which type of reaction is heat added to the reactants?
- In which type of reaction is heat produced?
- If there was no energy added to an endothermic reaction, would that reaction occur? Why or why not?
- If there was no energy added to an exothermic reaction, would that reaction occur? Why or why not?
- Explain why a chemical cold pack feels cold when activated.
- Explain why cellular respiration and photosynthesis are “opposites” of each other.
- Explain how the sun gives our cells energy indirectly.
3.9 Explore More
Activation energy: Kickstarting chemical reactions – Vance Kite, TED-Ed, 2013.
The Sci Guys: Science at Home – SE1 – EP7: Hot Ice – Exothermic Reactions and Supercooled solutions, The Sci Guys, 2013
Attributions
Figure 3.9.1
Rusty truck by Ross Sokolovski on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 3.9.2
Compost/ Gently steaming compost! by John Winfield on Wikimedia Commons, is used under a
Figure 3.9.3
Cold Pack by OpenStax /CNX on Wikimedia Commons, is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
Figure 3.9.4
Activation energy by CK12 is used under a CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/) license.
References
Brainard, J., Henderson, R. / CK12. (2018, August 22). Figure: Activation Energy [digital image]. In CK-12 College Human Biology. CK12. https://flexbooks.ck12.org/cbook/ck-12-college-human-biology-flexbook-2.0
OpenStax. (2019, Jul 30), Figure 6(b) A cold pack uses an endothermic process to create the sensation of cold. OpenStax Chemistry. OpenStax CNX. http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@12.2. (Credit: a modification of work by “Skatebiker”/Wikimedia commons).
TED-Ed. (2013, January 9). Activation energy: Kickstarting chemical reactions – Vance Kite. YouTube. https://www.youtube.com/watch?v=D0ZyjpAin_Y&feature=youtu.be
The Sci Guys. (2013, April 4). The Sci Guys: Science at home – SE1 – EP7: Hot ice – Exothermic reactions and supercooled solutions. YouTube. https://www.youtube.com/watch?v=znsPa1BSaIM&feature=youtu.be
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another.
The ability to do work.
A chemical reaction that releases energy through light or heat.
Created by: CK-12/Adapted by Christine Miller

Bring on the S'mores!
This inviting camp fire can be used for both heat and light. Heat and light are two forms of energy that are released when a fuel like wood is burned. The cells of living things also get energy by "burning." They "burn" glucose in a process called cellular respiration.
What Is Cellular Respiration?
Cellular respiration is the process by which living cells break down glucose molecules and release energy. The process is similar to burning, although it doesn’t produce light or intense heat as a campfire does. This is because cellular respiration releases the energy in glucose slowly and in many small steps. It uses the energy released to form molecules of ATP, the energy-carrying molecules that cells use to power biochemical processes. In this way, cellular respiration is an example of energy coupling: glucose is broken down in an exothermic reaction, and then the energy from this reaction powers the endothermic reaction of the formation of ATP. Cellular respiration involves many chemical reactions, but they can all be summed up with this chemical equation:
C6H12O6 6O2 → 6CO2 6H2O Chemical Energy (in ATP)
In words, the equation shows that glucose (C6H12O6) and oxygen (O2) react to form carbon dioxide (CO2) and water (H2O), releasing energy in the process. Because oxygen is required for cellular respiration, it is an aerobic process.
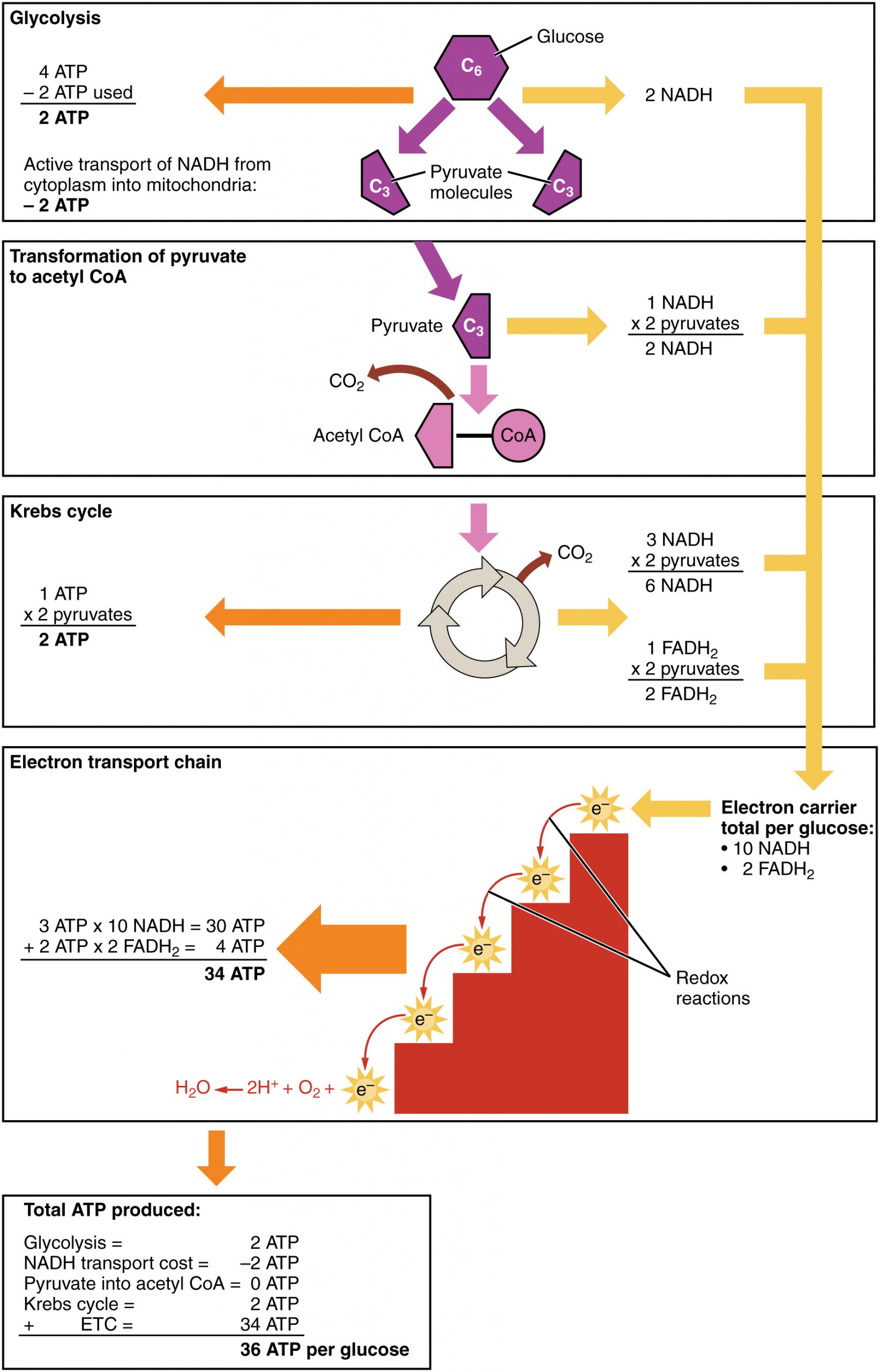
Cellular respiration occurs in the cells of all living things, both autotrophs and heterotrophs. All of them burn glucose to form ATP. The reactions of cellular respiration can be grouped into three stages: glycolysis, the Krebs cycle (also called the citric acid cycle), and electron transport. Figure 4.10.2 gives an overview of these three stages, which are also described in detail below.
Cellular Respiration Stage I: Glycolysis
The first stage of cellular respiration is glycolysis, which happens in the cytosol of the cytoplasm.
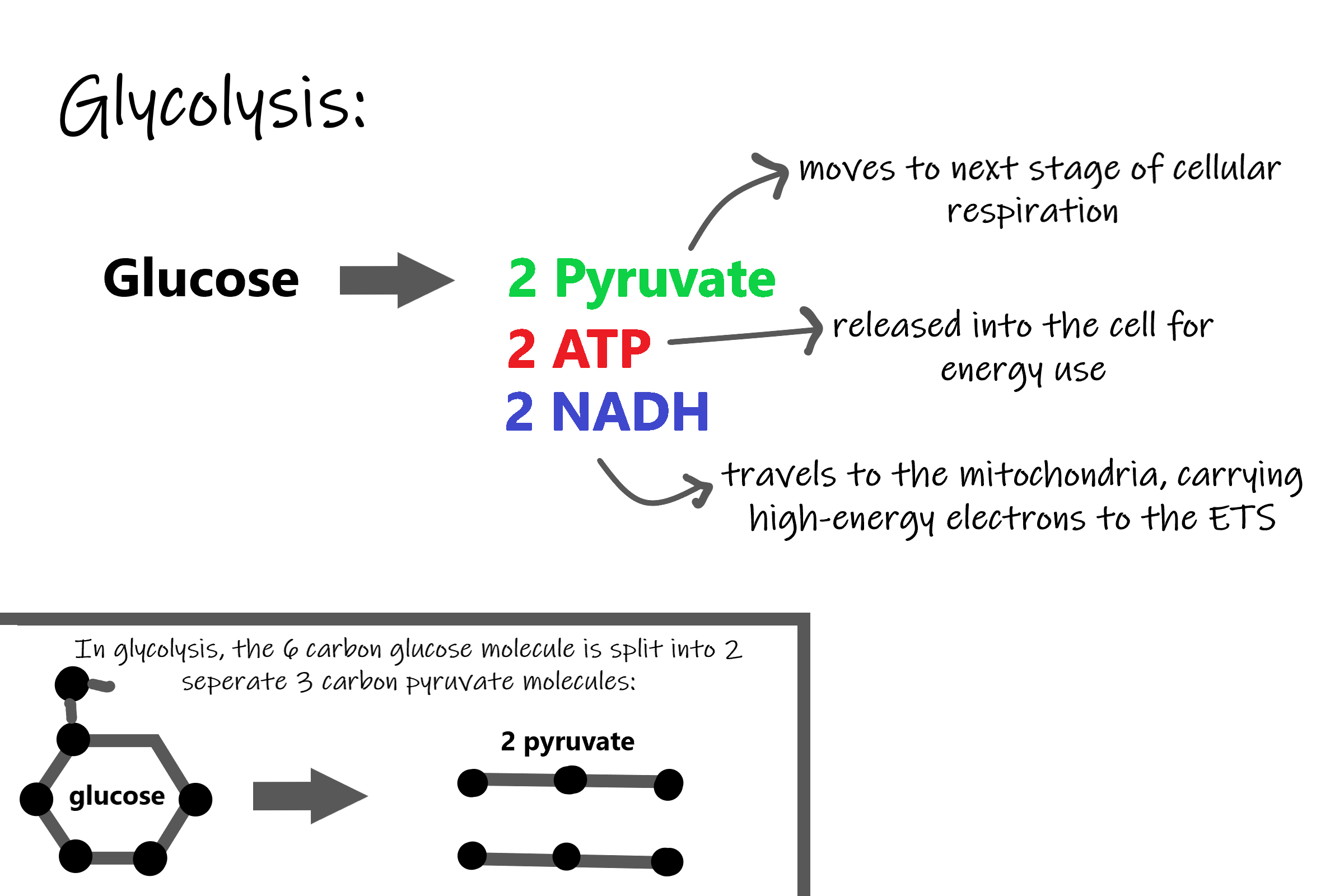
Splitting Glucose
The word glycolysis literally means “glucose splitting,” which is exactly what happens in this stage. Enzymes split a molecule of glucose into two molecules of pyruvate (also known as pyruvic acid). This occurs in several steps, as summarized in the following diagram.
Results of Glycolysis
Energy is needed at the start of glycolysis to split the glucose molecule into two pyruvate molecules which go on to stage II of cellular respiration. The energy needed to split glucose is provided by two molecules of ATP; this is called the energy investment phase. As glycolysis proceeds, energy is released, and the energy is used to make four molecules of ATP; this is the energy harvesting phase. As a result, there is a net gain of two ATP molecules during glycolysis. During this stage, high-energy electrons are also transferred to molecules of NAD to produce two molecules of NADH, another energy-carrying molecule. NADH is used in stage III of cellular respiration to make more ATP.
Transition Reaction
Before pyruvate can enter the next stage of cellular respiration it needs to be modified slightly. The transition reaction is a very short reaction which converts the two molecules of pyruvate to two molecules of acetyl CoA, carbon dioxide, and two high energy electron pairs convert NAD to NADH. The carbon dioxide is released, the acetyl CoA moves to the mitochondria to enter the Kreb's Cycle (stage II), and the NADH carries the high energy electrons to the Electron Transport System (stage III).
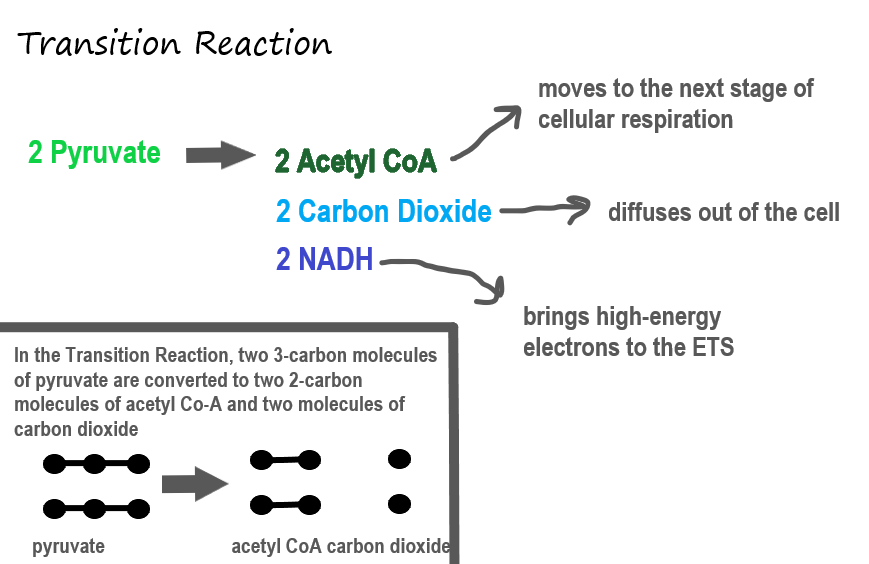
Structure of the Mitochondrion
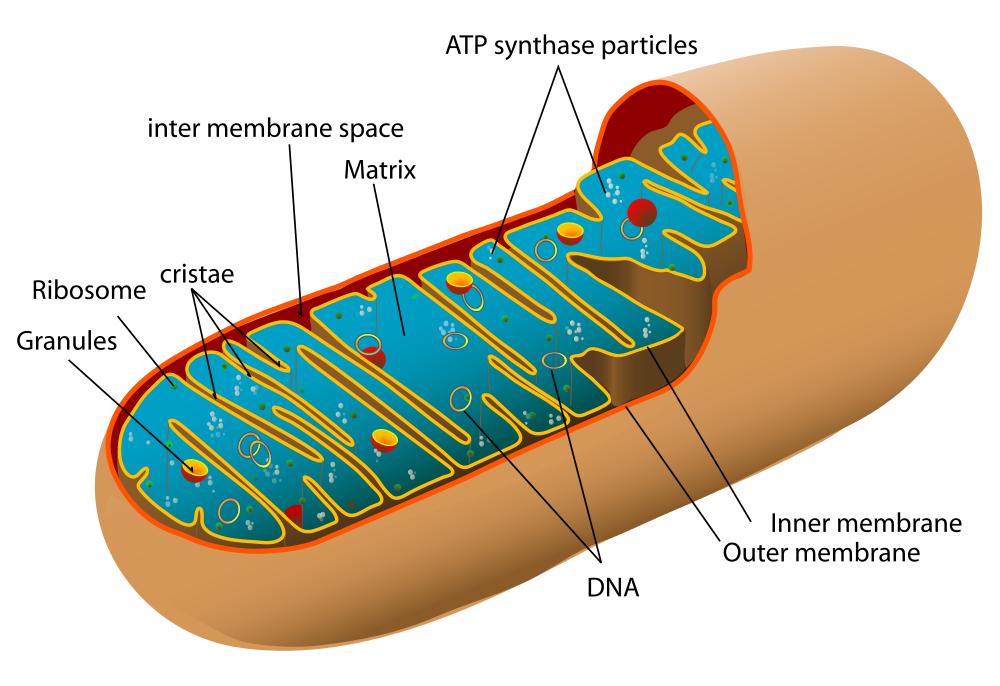
Before you read about the last two stages of cellular respiration, you need to know more about the mitochondrion, where these two stages take place. A diagram of a mitochondrion is shown in Figure 4.10.5.
The structure of a mitochondrion is defined by an inner and outer membrane. This structure plays an important role in aerobic respiration.
As you can see from the figure, a mitochondrion has an inner and outer membrane. The space between the inner and outer membrane is called the intermembrane space. The space enclosed by the inner membrane is called the matrix. The second stage of cellular respiration (the Krebs cycle) takes place in the matrix. The third stage (electron transport) happens on the inner membrane.
Cellular Respiration Stage II: The Krebs Cycle
Recall that glycolysis produces two molecules of pyruvate (pyruvic acid), which are then converted to acetyl CoA during the short transition reaction. These molecules enter the matrix of a mitochondrion, where they start the Krebs cycle (also known as the Citric Acid Cycle). The reason this stage is considered a cycle is because a molecule called oxaloacetate is present at both the beginning and end of this reaction and is used to break down the two molecules of acetyl CoA. The reactions that occur next are shown in Figure 4.10.6.
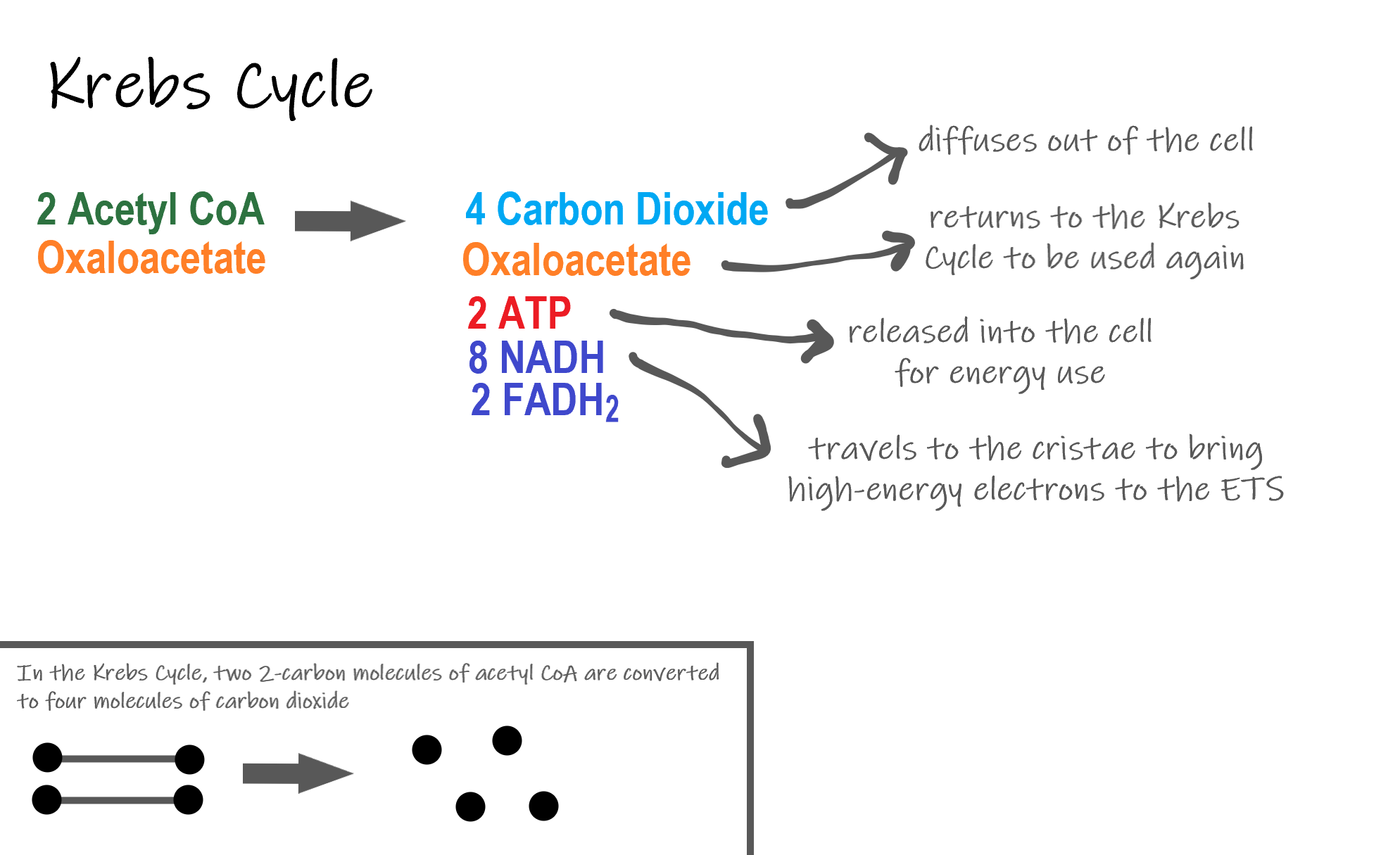
Steps of the Krebs Cycle
The Krebs cycle itself actually begins when acetyl-CoA combines with a four-carbon molecule called OAA (oxaloacetate) (see Figure 4.10.6). This produces citric acid, which has six carbon atoms. This is why the Krebs cycle is also called the citric acid cycle.
After citric acid forms, it goes through a series of reactions that release energy. The energy is captured in molecules of NADH, ATP, and FADH2, another energy-carrying coenzyme. Carbon dioxide is also released as a waste product of these reactions.
The final step of the Krebs cycle regenerates OAA, the molecule that began the Krebs cycle. This molecule is needed for the next turn through the cycle. Two turns are needed because glycolysis produces two pyruvic acid molecules when it splits glucose.
Results of the Glycolysis, Transition Reaction and Krebs Cycle
After glycolysis, transition reaction, and the Krebs cycle, the glucose molecule has been broken down completely. All six of its carbon atoms have combined with oxygen to form carbon dioxide. The energy from its chemical bonds has been stored in a total of 16 energy-carrier molecules. These molecules are:
- 4 ATP (2 from glycolysis, 2 from Krebs Cycle)
- 12 NADH (2 from glycolysis, 2 from transition reaction, and 8 from Krebs cycle)
- 2 FADH2 (both from the Krebs cycle)
The events of cellular respiration up to this point are exergonic reactions- they are releasing energy that had been stored in the bonds of the glucose molecule. This energy will be transferred to the third and final stage of cellular respiration: the Electron Transport System, which is an endergonic reaction. Using an exothermic reaction to power an endothermic reaction is known as energy coupling.
Cellular Respiration Stage III: Electron Transport Chain
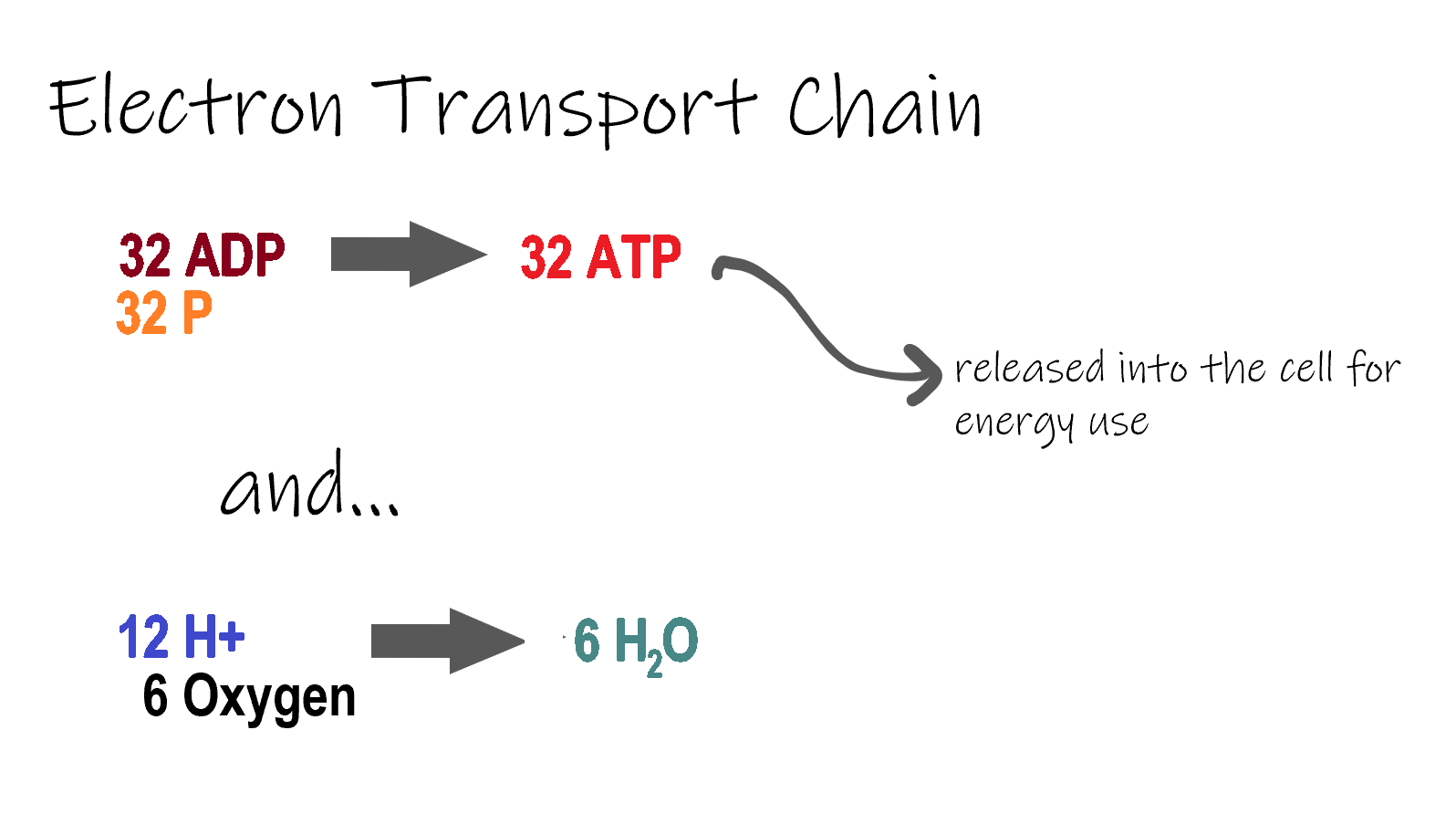
ETC, the final stage in cellular respiration produces 32 ATP. The Electron Transport Chain is the final stage of cellular respiration. In this stage, energy being transported by NADH and FADH2 is transferred to ATP. In addition, oxygen acts as the final proton acceptor for the hydrogens released from all the NADH and FADH2, forming water. Figure 4.10.8 shows the reactants and products of the ETC.
Transporting Electrons
The Electron transport chain is the third stage of cellular respiration and is illustrated in Figure 4.10.8. During this stage, high-energy electrons are released from NADH and FADH2, and they move along electron-transport chains on the inner membrane of the mitochondrion. An electron-transport chain is a series of molecules that transfer electrons from molecule to molecule by chemical reactions. Some of the energy from the electrons is used to pump hydrogen ions (H ) across the inner membrane, from the matrix into the intermembrane space. This ion transfer creates an electrochemical gradient that drives the synthesis of ATP.
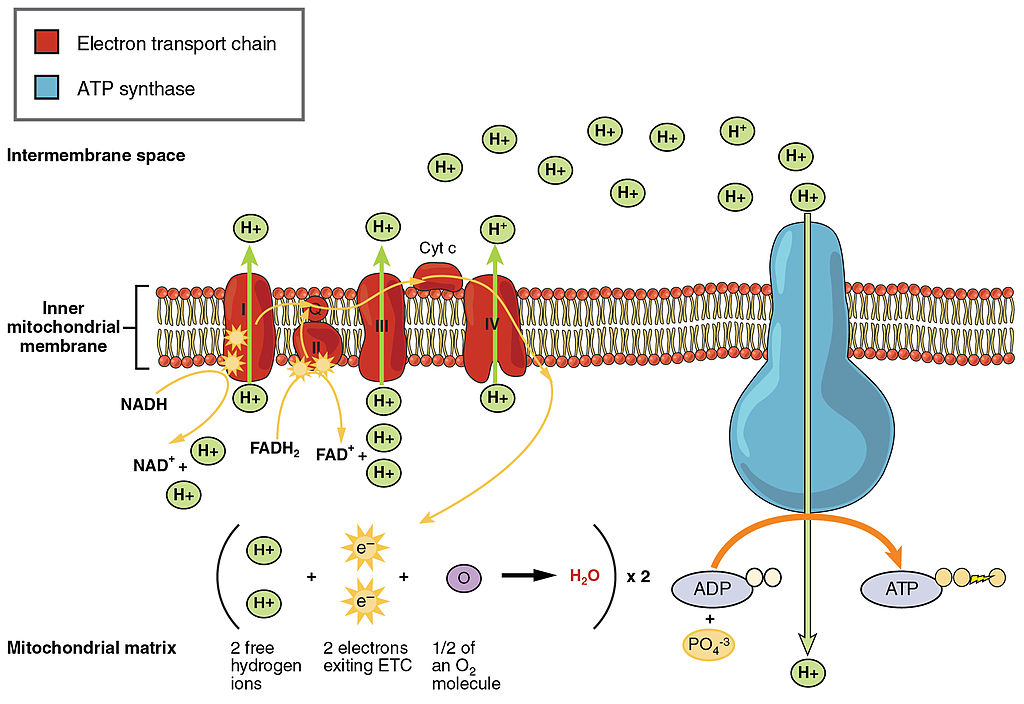
Making ATP
As shown in Figure 4.10.8, the pumping of hydrogen ions across the inner membrane creates a greater concentration of the ions in the intermembrane space than in the matrix. This gradient causes the ions to flow back across the membrane into the matrix, where their concentration is lower. ATP synthase acts as a channel protein, helping the hydrogen ions cross the membrane. It also acts as an enzyme, forming ATP from ADP and inorganic phosphate in a process called oxidative phosphorylation. After passing through the electron-transport chain, the “spent” electrons combine with oxygen to form water.
How Much ATP?
You have seen how the three stages of aerobic respiration use the energy in glucose to make ATP. How much ATP is produced in all three stages combined? Glycolysis produces two ATP molecules, and the Krebs cycle produces two more. Electron transport begins with several molecules of NADH and FADH2 from the Krebs cycle and transfers their energy into as many as 34 more ATP molecules. All told, then, up to 38 molecules of ATP can be produced from just one molecule of glucose in the process of cellular respiration.
4.10 Summary
- Cellular respiration is the aerobic process by which living cells break down glucose molecules, release energy, and form molecules of ATP. Generally speaking, this three-stage process involves glucose and oxygen reacting to form carbon dioxide and water.
- The first stage of cellular respiration, called glycolysis, takes place in the cytoplasm. In this step, enzymes split a molecule of glucose into two molecules of pyruvate, which releases energy that is transferred to ATP. Following glycolysis, a short reaction called the transition reaction converts the pyruvate into two molecules of acetyl CoA.
- The organelle called a mitochondrion is the site of the other two stages of cellular respiration. The mitochondrion has an inner and outer membrane separated by an intermembrane space, and the inner membrane encloses a space called the matrix.
- The second stage of cellular respiration, called the Krebs cycle, takes place in the matrix of a mitochondrion. During this stage, two turns through the cycle result in all of the carbon atoms from the two pyruvate molecules forming carbon dioxide and the energy from their chemical bonds being stored in a total of 16 energy-carrying molecules (including two from glycolysis and two from transition reaction).
- The third and final stage of cellular respiration, called electron transport, takes place on the inner membrane of the mitochondrion. Electrons are transported from molecule to molecule down an electron-transport chain. Some of the energy from the electrons is used to pump hydrogen ions across the membrane, creating an electrochemical gradient that drives the synthesis of many more molecules of ATP.
- In all three stages of cellular respiration combined, as many as 38 molecules of ATP are produced from just one molecule of glucose.
4.10 Review Questions
- What is the purpose of cellular respiration? Provide a concise summary of the process.
- State what happens during glycolysis.
- Describe the structure of a mitochondrion.
- What molecule is present at both the beginning and end of the Krebs cycle?
- What happens during the electron transport stage of cellular respiration?
- How many molecules of ATP can be produced from one molecule of glucose during all three stages of cellular respiration combined?
- Do plants undergo cellular respiration? Why or why not?
- Explain why the process of cellular respiration described in this section is considered aerobic.
- Name three energy-carrying molecules involved in cellular respiration.
-
- Which stage of aerobic cellular respiration produces the most ATP?
-
4.10 Explore More
https://www.youtube.com/watch?time_continue=2&v=00jbG_cfGuQ&feature=emb_logo
ATP & Respiration: Crash Course Biology #7, CrashCourse, 2012.
https://www.youtube.com/watch?v=4Eo7JtRA7lg&t=3s
Cellular Respiration and the Mighty Mitochondria, The Amoeba Sisters, 2014.
Attributions
Figure 4.10.1
Smores by Jessica Ruscello on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 4.10.2
Carbohydrate_Metabolism by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 4.10.3
Glycolysis by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.4
Transition Reaction by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.5
Mitochondrion by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.10.6
Krebs cycle by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.7
Electron Transport Chain (ETC) by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.8
The_Electron_Transport_Chain by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
References
CrashCourse. (2012, March 12). ATP & Respiration: Crash Course Biology #7. YouTube. https://www.youtube.com/watch?time_continue=2&v=00jbG_cfGuQ&feature=emb_logo
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 24.8 Electron Transport Chain [digital image]. In Anatomy & Physiology, Connexions (Section ). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/24-2-carbohydrate-metabolism
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 24.9 Carbohydrate Metabolism [digital image]. In Anatomy & Physiology, Connexions (Section 24.2). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/24-2-carbohydrate-metabolism
The Amoeba Sisters. (2014, October 22). Cellular Respiration and the Mighty Mitochondria. YouTube. https://www.youtube.com/watch?v=4Eo7JtRA7lg&t=3s
Any reaction which requires or absorbs energy from its surroundings, usually in the form of heat.
The minimum energy required to cause a reaction to occur.
Created by CK-12 Foundation/Adapted by Christine Miller

Double Jointed?
Is this woman double jointed? No, there is actually no such thing — at least as far as humans are concerned. However, some people, like the woman pictured in Figure 11.6.1, are much more flexible than others, generally because they have looser ligaments. Physicians call this condition joint hypermobility. Regardless of what it’s called, the feats of people with highly mobile joints can be quite impressive.
What Are Joints?
Joints are locations at which bones of the skeleton connect with one another. A joint is also called an articulation. The majority of joints are structured in such a way that they allow movement. However, not all joints allow movement. Of joints that do allow movement, the extent and direction of the movements they allow also vary.
Classification of Joints
Joints can be classified structurally or functionally. The structural classification of joints depends on the manner in which the bones connect to each other. The functional classification of joints depends on the nature of the movement the joints allow. There is significant overlap between the two types of classifications, because function depends largely on structure.
Structural Classification of Joints
The structural classification of joints is based on the type of tissue that binds the bones to each other at the joint. There are three types of joints in the structural classification: fibrous, cartilaginous, and synovial joints.
- Fibrous joints are joints in which bones are joined by dense connective tissue that is rich in collagen fibres. These joints are also called sutures. The joints between bones of the cranium are fibrous joints.
- Cartilaginous joints are joints in which bones are joined by cartilage. The joints between most of the vertebrae in the spine are cartilaginous joints.
- Synovial joints are characterized by a fluid-filled space (called a synovial cavity) between the bones of the joints. You can see a drawing of a typical synovial joint in Figure 11.6.2. The cavity is enclosed by a membrane and filled with a fluid (called synovial fluid) that provides extra cushioning to the ends of the bones. Cartilage covers the articulating surfaces of the two bones, but the bones are actually held together by ligaments. The knee is a synovial joint.
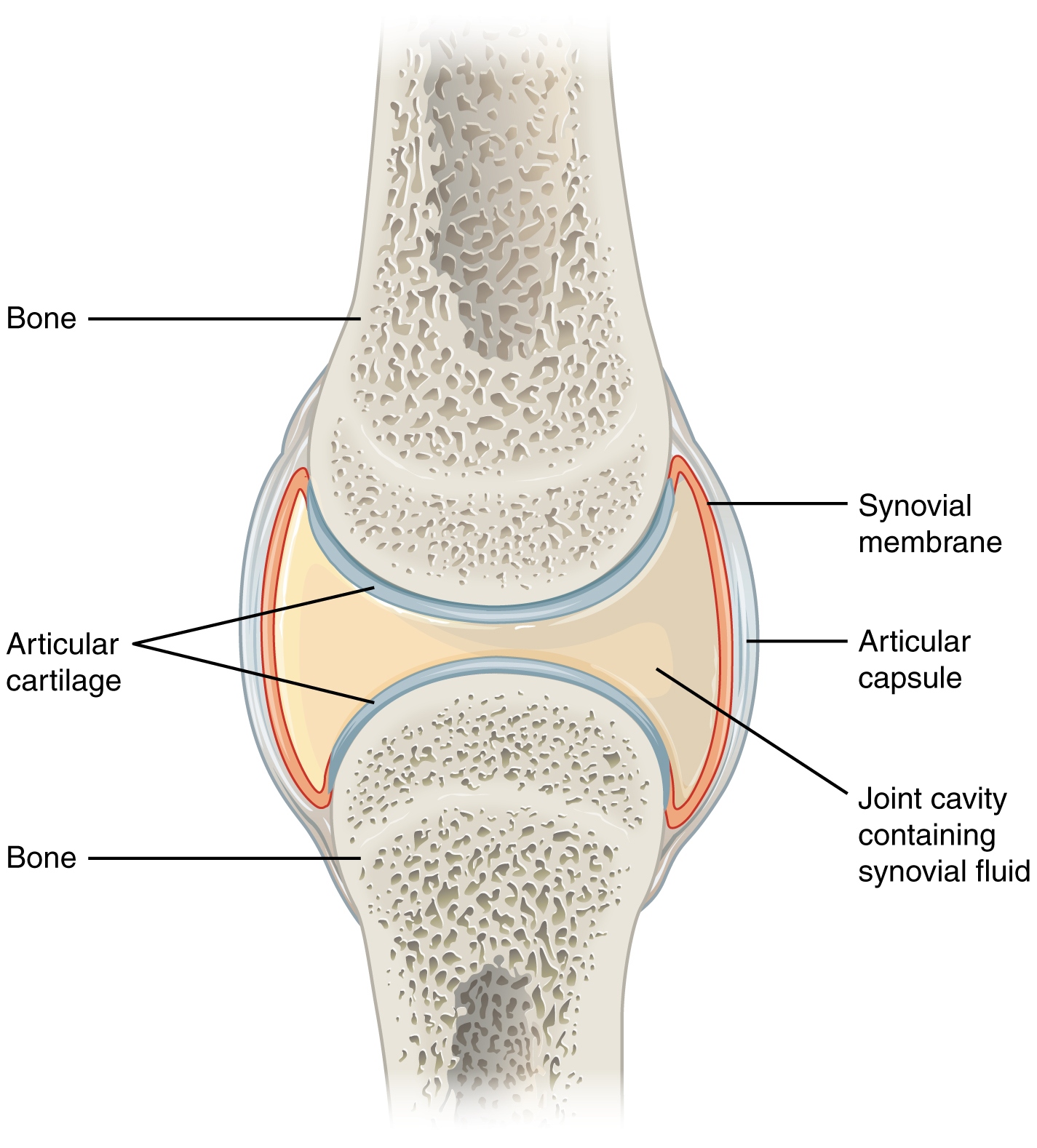
Functional Classification of Joints
The functional classification of joints is based on the type and degree of movement that they allow. There are three types of joints in the functional classification: immovable, partly movable, and movable joints.
- Immovable joints allow little or no movement at the joint. Most immovable joints are fibrous joints. Besides the bones of the cranium, immovable joints include joints between the tibia and fibula in the lower leg, and between the radius and ulna in the lower arm.
- Partly movable joints permit slight movement. Most partly movable joints are cartilaginous joints. Besides the joints between vertebrae, they include the joints between the ribs and sternum (breast bone).
- Movable joints allow bones to move freely. All movable joints are synovial joints. Besides the knee, they include the shoulder, hip, and elbow. Movable joints are the most common type of joints in the body.
Types of Movable Joints
Movable joints can be classified further according to the type of movement they allow. There are six classes of movable joints: pivot, hinge, saddle, plane, condyloid, and ball-and-socket joints. An example of each class — as well as the type of movement it allows — is shown in Figure 11.6.3.
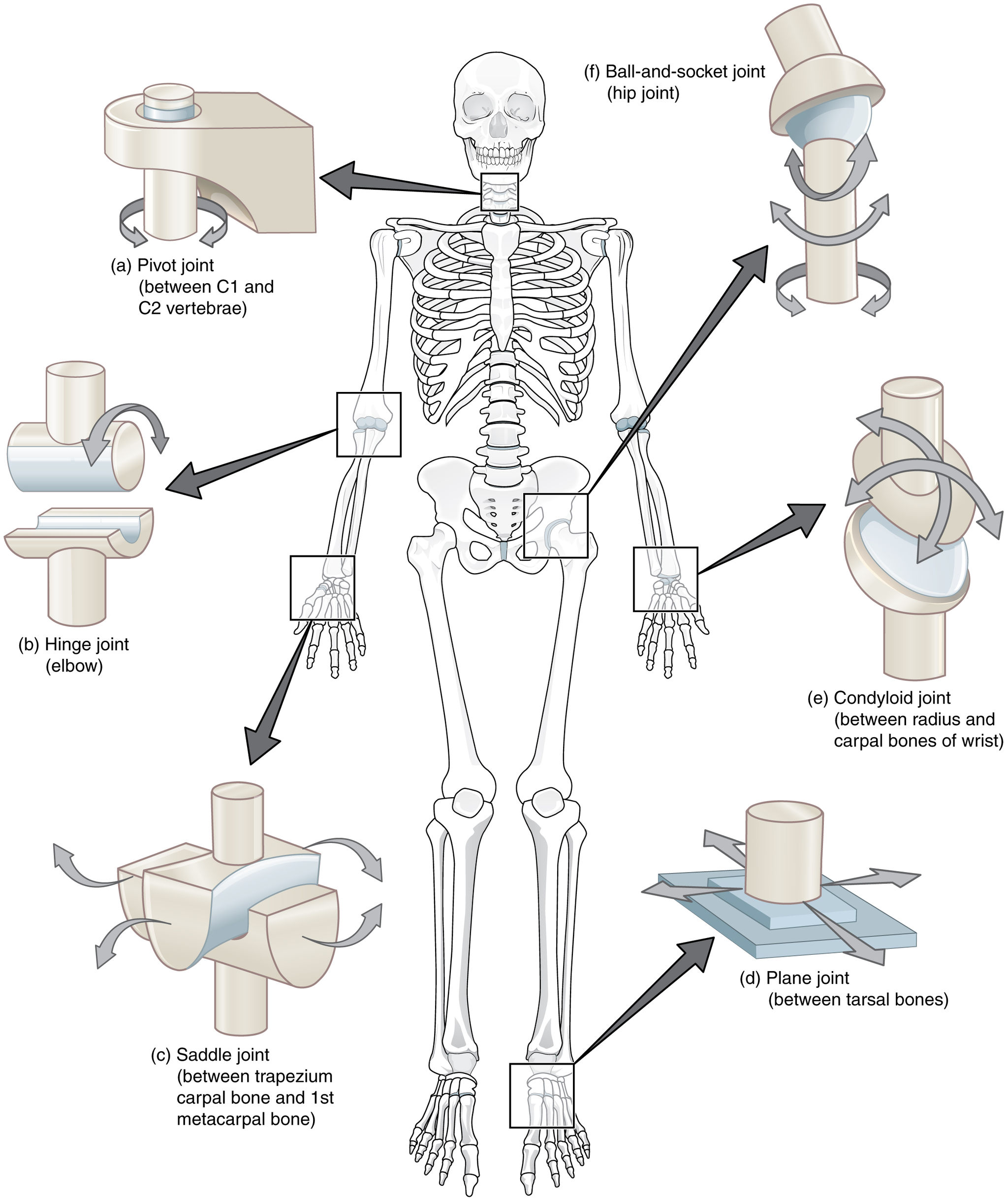
- A ball-and-socket joint allows the greatest range of movement of any movable joint. It allows forward and backward motion, as well as upward and downward movement. It also allows rotation in a circle. The hip and shoulder are the only two ball-and-socket joints in the human body.
- A pivot joint allows one bone to rotate around another. An example of a pivot joint is the joint between the first two vertebrae in the spine. This joint allows the head to rotate from left to right and back again.
- A hinge joint allows back and forth movement like the hinge of a door. An example of a hinge joint is the elbow. This joint allows the arm to bend back and forth.
- A saddle joint allows two different types of movement. An example of a saddle joint is the joint between the first metacarpal bone in the hand and one of the carpal bones in the wrist. This joint allows the thumb to move toward and away from the index finger, and also to cross over the palm toward the little finger.
- A plane joint (also called a gliding joint) allows two bones to glide over one another. The joints between the tarsals in the ankles and between the carpals in the wrists are mainly gliding joints. In the wrist, this type of joint allows the hand to bend upward at the wrist, and also to wave from side to side while the lower arm is held steady.
- A condyloid joint is one in which an oval-shaped head on one bone moves in an elliptical cavity in another bone, allowing movement in all directions, except rotation around an axis. The joint between the radius in the lower arm and carpal bones of the wrist is a condyloid joint, as is the joint at the base of the index finger.
Feature: My Human Body
Of all the parts of the skeletal system, the joints are generally the most fragile and subject to damage. If the cartilage that cushions bones at joints wears away, it does not grow back. Eventually, all of the cartilage may wear away. This causes osteoarthritis, which can be both painful and debilitating. In serious cases of osteoarthritis, people may lose the ability to climb stairs, walk long distances, perform routine daily activities, or participate in activities they love, such as gardening or playing sports. If you protect your joints, you can reduce your chances of joint damage, pain, and disability. If you already have joint damage, it is equally important to protect your joints and limit further damage. Follow these five tips:
- Maintain a normal, healthy weight. The more you weigh, the more force you exert on your joints. When you walk, each knee has to bear a force equal to as much as six times your body weight. If a person weighs 200 pounds, each knee bears more than half a ton of weight with every step. Seven in ten knee replacement surgeries for osteoarthritis can be attributed to obesity.
- Avoid too much high-impact exercise. Examples of high-impact activities include volleyball, basketball, and tennis. These activities generally involve running or jumping on hard surfaces, which puts tremendous stress on weight-bearing joints, especially the knees. Replace some or all of your high-impact activities with low-impact activities, such as biking, swimming, yoga, or lifting light weights.
- Reduce your risk of injury. Don’t be a weekend warrior, sitting at a desk all week and then crowding all your physical activity into two days. Get involved in a regular, daily exercise routine that keeps your body fit and your muscles toned. Building up muscles will make your joints more stable, allowing stress to spread across them. Be sure to do some stretching every day to keep the muscles around joints flexible and less prone to injury.
- Distribute work over your body, and use your largest, strongest joints. Use your shoulder, elbow, and wrist to lift heavy objects — not just your fingers. Hold small items in the palm of your hand, rather than by the fingers. Carry heavy items in a backpack, rather than in your hands. Hold weighty objects close to your body, instead of at arms’ length. Lift with your hips and knees, not your back.
- Respect pain. If it hurts, stop doing it. Take a break from the activity — at least until the pain stops. Try to use joints only to the point of mild fatigue, not pain.
11.6 Summary
- Joints are spots at which bones of the skeleton connect with one another. A joint is also called an articulation.
- Joints can be classified structurally or functionally, and there is significant overlap between the two types of classifications.
- The structural classification of joints depends on the type of tissue that binds the bones to each other at the joint. There are three types of joints in the structural classification: fibrous, cartilaginous, and synovial joints.
- The functional classification of joints is based on the type and degree of movement that they allow. There are three types of joints in the functional classification: immovable, partly movable, and movable joints.
- Movable joints can be classified further according to the type of movement they allow. There are six classes of movable joints: pivot, hinge, saddle, plane, condyloid, and ball-and-socket joints.
11.6 Review Questions
- What are joints?
- What are two ways that joints are commonly classified?
-
- How are joints classified structurally?
- Describe the functional classification of joints.
- How are movable joints classified?
- Name the six classes of movable joints. Describe how they move and give an example of each.
- Which specific type of moveable joint do you think your knee joint is? Explain your reasoning.
- Explain the difference between cartilage in a cartilaginous joint and cartilage in a synovial joint.
- Why are fibrous joints immovable?
- What is the function of synovial fluid?
11.6 Explore More
https://www.youtube.com/watch?v=IjiKUmfaZr4
Why do your knuckles pop? - Eleanor Nelsen, TED-Ed, 2015.
https://www.youtube.com/watch?v=FWsBm3hr3B0
Why haven’t we cured arthritis? - Kaitlyn Sadtler and Heather J. Faust, TED-Ed, 2019.
Attributions
Figure 11.6.1
Tags: Sports Gymnastics Fitness Woman Preparation by nastya_gepp on Pixabay is used under the Pixabay License (https://pixabay.com/de/service/license/).
Figure 11.6.2
Synovial_Joints by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 11.6.3
Types_of_Synovial_Joints by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
References
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, June 19). Figure 9.8 Synovial joints [digital image]. In Anatomy and Physiology (Section 9.4). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/9-4-synovial-joints
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, June 19). Figure 9.10 Types of synovial joints [digital image]. In Anatomy and Physiology (Section 9.4). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/9-4-synovial-joints
Mayo Clinic Staff. (n.d.). Osteoarthritis [online article]. MayoClinic.org. https://www.mayoclinic.org/diseases-conditions/osteoarthritis/symptoms-causes/syc-20351925
TED-Ed. (2015, May 5). Why do your knuckles pop? - Eleanor Nelsen. YouTube. https://www.youtube.com/watch?v=IjiKUmfaZr4
TED-Ed. (2019, November 7). Why haven’t we cured arthritis? - Kaitlyn Sadtler and Heather J. Faust. YouTube. https://www.youtube.com/watch?v=FWsBm3hr3B0

