14.5 Blood

Vampires
From Bram Stoker’s famous novel about Count Dracula, to films such as Van Helsing and the Twilight Saga, fantasies featuring vampires (like the one in Figure 14.5.1) have been popular for decades. Vampires, in fact, are found in centuries-old myths from many cultures. In such myths, vampires are generally described as creatures that drink blood — preferably of the human variety — for sustenance. Dracula, for example, is based on Eastern European folklore about a human who attains immortality (and eternal damnation) by drinking the blood of others.
What Is Blood?
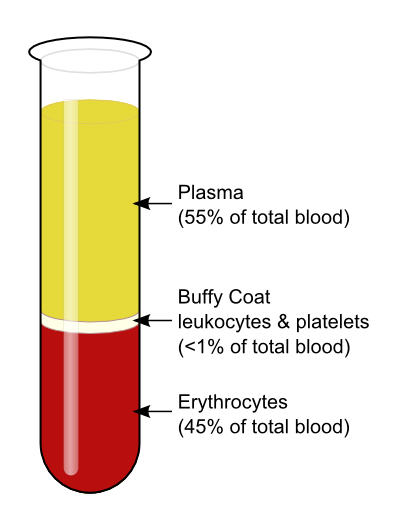
The average adult body contains between 4.7 and 5.7 litres of blood. More than half of that amount is fluid. Most of the rest of that amount consists of blood cells. The relative amounts of the various components in blood are illustrated in Figure 14.5.2. The components are also described in detail below.
Blood is a fluid connective tissue that circulates throughout the body through blood vessels of the cardiovascular system. What makes blood so special that it features in widespread myths? Although blood accounts for less than 10% of human body weight, it is quite literally the elixir of life. As blood travels through the vessels of the cardiovascular system, it delivers vital substances (such as nutrients and oxygen) to all of the cells, and carries away their metabolic wastes. It is no exaggeration to say that without blood, cells could not survive. Indeed, without the oxygen carried in blood, cells of the brain start to die within a matter of minutes.
Functions of Blood
Blood performs many important functions in the body. Major functions of blood include:
- Supplying tissues with oxygen, which is needed by all cells for aerobic cellular respiration.
- Supplying cells with nutrients, including glucose, amino acids, and fatty acids.
- Removing metabolic wastes from cells, including carbon dioxide, urea, and lactic acid.
- Helping to defend the body from pathogens and other foreign substances.
- Forming clots to seal broken blood vessels and stop bleeding.
- Transporting hormones and other messenger molecules.
- Regulating the pH of the body, which must be kept within a narrow range (7.35 to 7.45).
- Helping regulate body temperature (through vasoconstriction and vasodilation).
Blood Plasma
Plasma is the liquid component of human blood. It makes up about 55% of blood by volume. It is about 92% water, and contains many dissolved substances. Most of these substances are proteins, but plasma also contains trace amounts of glucose, mineral ions, hormones, carbon dioxide, and other substances. In addition, plasma contains blood cells. When the cells are removed from plasma, as in Figure 14.5.2 above, the remaining liquid is clear but yellow in colour.
Blood Cells
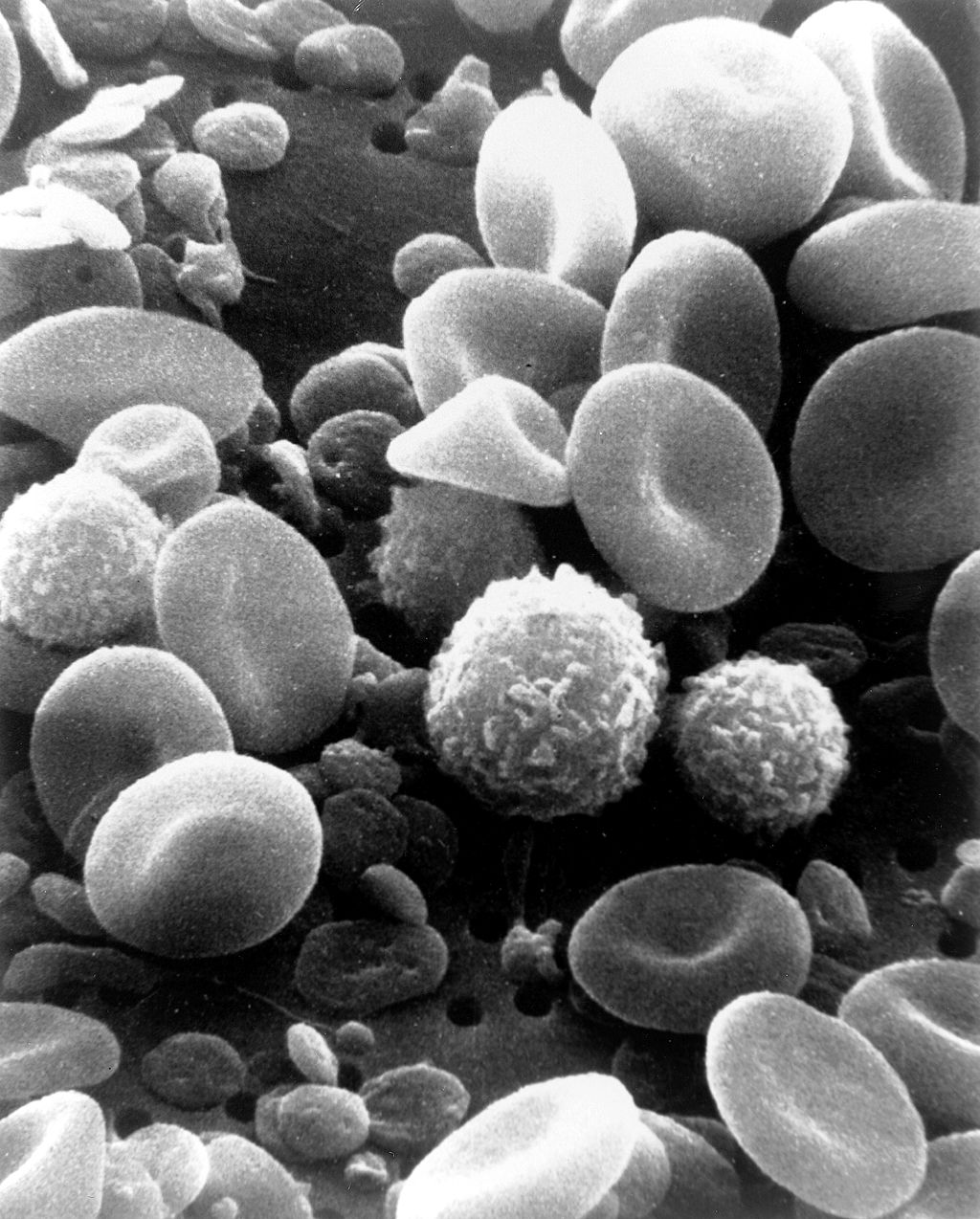
The cells in blood include erythrocytes, leukocytes, and thrombocytes. These different types of blood cells are shown in the photomicrograph (Figure 14.5.3) and described in the sections that follow.
Erythrocytes
The most numerous cells in blood are red blood cells, also called erythrocytes. One microlitre of blood contains between 4.2 and 6.1 million red blood cells, and red blood cells make up about 25% of all the cells in the human body. The cytoplasm of a mature erythrocyte is almost completely filled with hemoglobin, the iron-containing protein that binds with oxygen and gives the cell its red colour. In order to provide maximum space for hemoglobin, mature erythrocytes lack a cell nucleus and most organelles. They are little more than sacks of hemoglobin.
Erythrocytes also carry proteins called antigens that determine blood type. Blood type is a genetic characteristic. The best known human blood type systems are the ABO and Rhesus systems.
- In the ABO system, there are two common antigens, called antigen A and antigen B. There are four ABO blood types, A (only A antigen), B (only B antigen), AB (both A and B antigens), and O (neither A nor B antigen). The ABO antigens are illustrated in Figure 14.5.4.
- In the Rhesus system, there is just one common antigen. A person may either have the antigen (Rh+) or lack the antigen (Rh-).
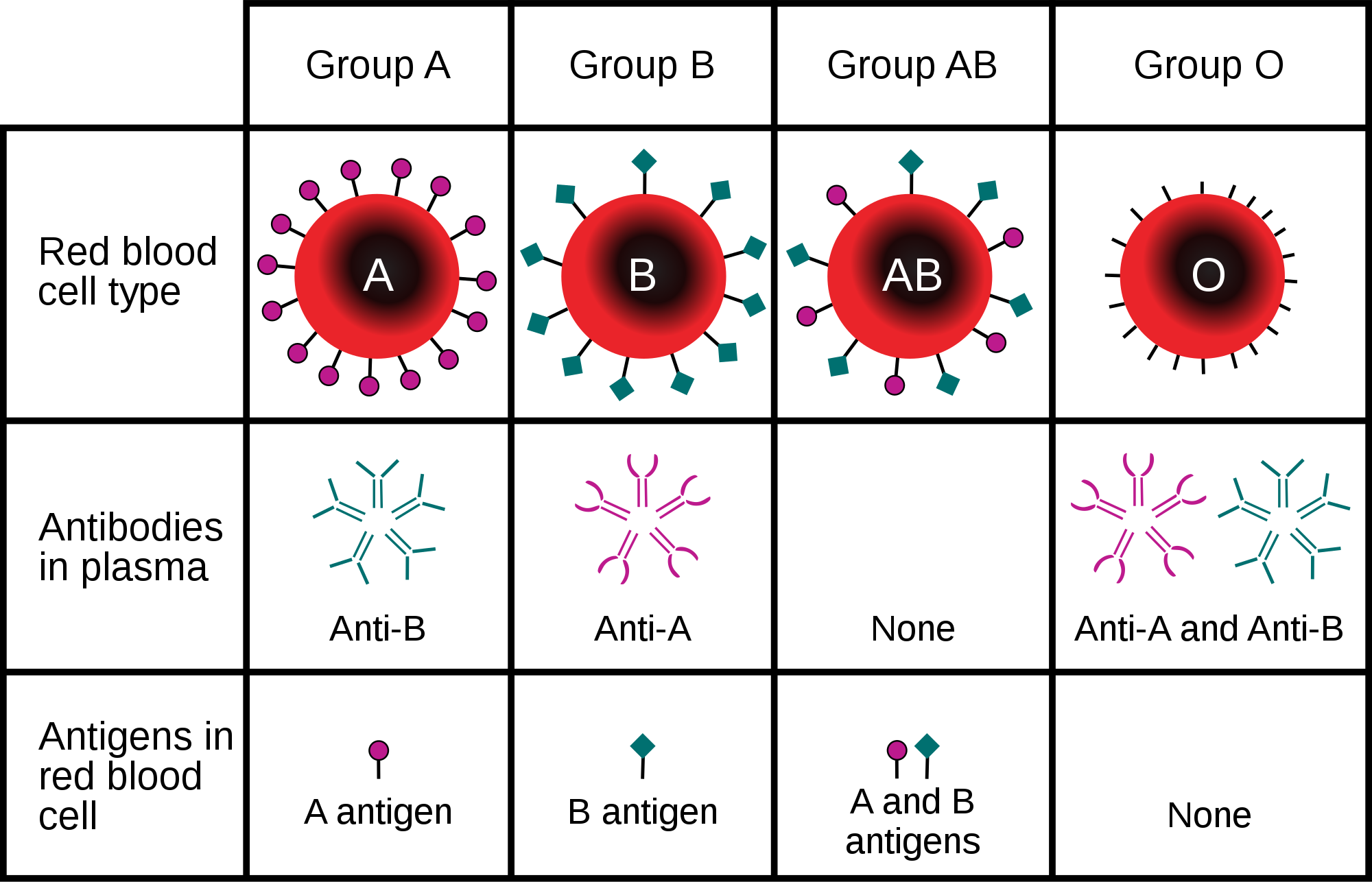
Blood type is important for medical reasons. A person who needs a blood transfusion must receive blood of a compatible type. Blood that is compatible lacks antigens that the patient’s own blood also lacks. For example, for a person with type A blood (no B antigen), compatible types include any type of blood that lacks the B antigen. This would include type A blood or type O blood, but not type AB or type B blood. If incompatible blood is transfused, it may cause a potentially life-threatening reaction in the patient’s blood.
Leukocytes
Leukocytes (also called white blood cells) are cells in blood that defend the body against invading microorganisms and other threats. There are far fewer leukocytes than red blood cells in blood. There are normally only about 1,000 to 11,000 white blood cells per microlitre of blood. Unlike erythrocytes, leukocytes have a nucleus. White blood cells are part of the body’s immune system. They destroy and remove old or abnormal cells and cellular debris, as well as attack pathogens and foreign substances. There are five main types of white blood cells, which are described in Table 14.5.1: neutrophils, eosinophils, basophils, lymphocytes, and monocytes. The five types differ in their specific immune functions.
| Type of Leukocyte | Per cent of All Leukocytes | Main Function(s) |
|---|---|---|
| Neutrophil | 62% | Phagocytize (engulf and destroy) bacteria and fungi in blood. |
| Eosinophil | 2% | Attack and kill large parasites; carry out allergic responses. |
| Basophil | less than 1% | Release histamines in inflammatory responses. |
| Lymphocyte | 30% | Attack and destroy virus-infected and tumor cells; create lasting immunity to specific pathogens. |
| Monocyte | 5% | Phagocytize pathogens and debris in tissues. |
Thrombocytes
Thrombocytes, also called platelets, are actually cell fragments. Like erythrocytes, they lack a nucleus and are more numerous than white blood cells. There are about 150 thousand to 400 thousand thrombocytes per microlitre of blood.
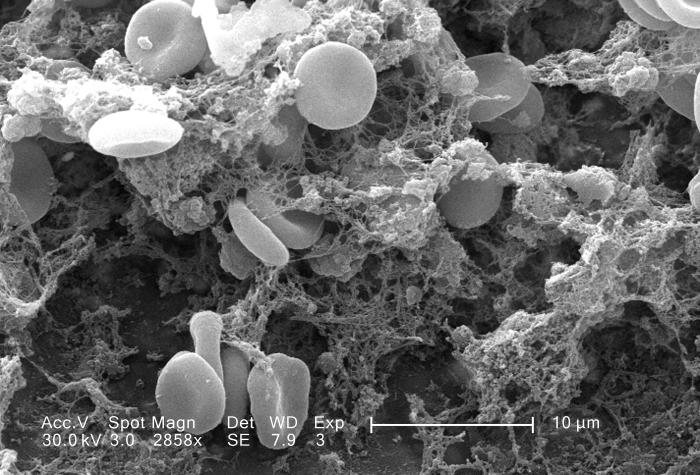
The main function of thrombocytes is blood clotting, or coagulation. This is the process by which blood changes from a liquid to a gel, forming a plug in a damaged blood vessel. If blood clotting is successful, it results in hemostasis, which is the cessation of blood loss from the damaged vessel. A blood clot consists of both platelets and proteins, especially the protein fibrin. You can see a scanning electron microscope photomicrograph of a blood clot in Figure 14.5.5.
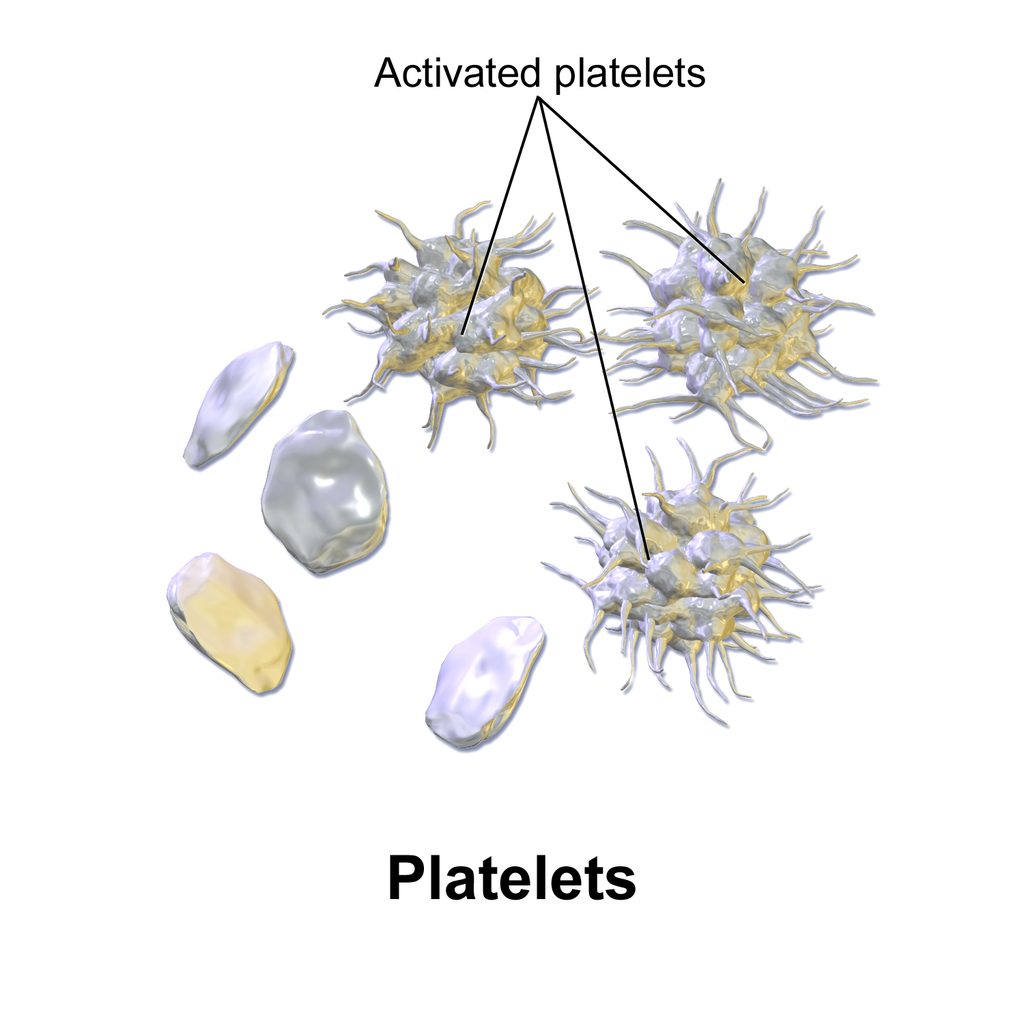
Coagulation begins almost instantly after an injury occurs to the endothelium of a blood vessel. Thrombocytes become activated and change their shape from spherical to star-shaped, as shown in Figure 14.5.6. This helps them aggregate with one another (stick together) at the site of injury to start forming a plug in the vessel wall. Activated thrombocytes also release substances into the blood that activate additional thrombocytes and start a sequence of reactions leading to fibrin formation. Strands of fibrin crisscross the platelet plug and strengthen it, much as rebar strengthens concrete.
Formation and Degradation of Blood Cells
Blood is considered a connective tissue, because blood cells form inside bones. All three types of blood cells are made in red marrow within the medullary cavity of bones in a process called hematopoiesis. Formation of blood cells occurs by the proliferation of stem cells in the marrow. These stem cells are self-renewing — when they divide, some of the daughter cells remain stem cells, so the pool of stem cells is not used up. Other daughter cells follow various pathways to differentiate into the variety of blood cell types. Once the cells have differentiated, they cannot divide to form copies of themselves.
Eventually, blood cells die and must be replaced through the formation of new blood cells from proliferating stem cells. After blood cells die, the dead cells are phagocytized (engulfed and destroyed) by white blood cells, and removed from the circulation. This process most often takes place in the spleen and liver.
Blood Disorders
Many human disorders primarily affect the blood. They include cancers, genetic disorders, poisoning by toxins, infections, and nutritional deficiencies.
- Leukemia is a group of cancers of the blood-forming tissues in the bone marrow. It is the most common type of cancer in children, although most cases occur in adults. Leukemia is generally characterized by large numbers of abnormal leukocytes. Symptoms may include excessive bleeding and bruising, fatigue, fever, and an increased risk of infections. Leukemia is thought to be caused by a combination of genetic and environmental factors.
- Hemophilia refers to any of several genetic disorders that cause dysfunction in the blood clotting process. People with hemophilia are prone to potentially uncontrollable bleeding, even with otherwise inconsequential injuries. They also commonly suffer bleeding into the spaces between joints, which can cause crippling.
- Carbon monoxide poisoning occurs when inhaled carbon monoxide (in fumes from a faulty home furnace or car exhaust, for example) binds irreversibly to the hemoglobin in erythrocytes. As a result, oxygen cannot bind to the red blood cells for transport throughout the body, and this can quickly lead to suffocation. Carbon monoxide is extremely dangerous, because it is colourless and odorless, so it cannot be detected in the air by human senses.
- HIV is a virus that infects certain types of leukocytes and interferes with the body’s ability to defend itself from pathogens and other causes of illness. HIV infection may eventually lead to AIDS (acquired immunodeficiency syndrome). AIDS is characterized by rare infections and cancers that people with a healthy immune system almost never acquire.
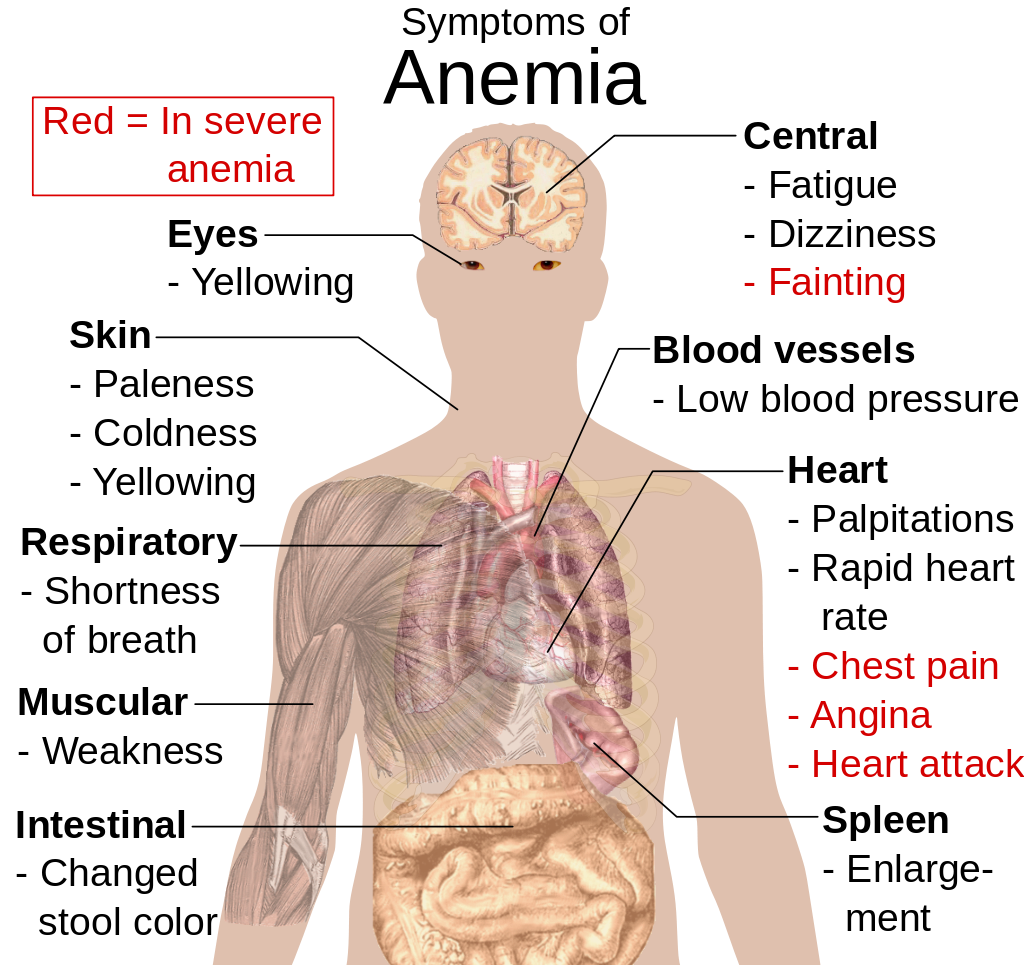
- Anemia is a disorder in which the blood has an inadequate volume of erythrocytes, reducing the amount of oxygen that the blood can carry, and potentially causing weakness and fatigue. These and other signs and symptoms of anemia are shown in Figure 14.5.8. Anemia has many possible causes, including excessive bleeding, inherited disorders (such as sickle cell hemoglobin), or nutritional deficiencies (iron, folate, or B12). Severe anemia may require transfusions of donated blood.
Feature: Myth vs. Reality
Donating blood saves lives. In fact, with each blood donation, as many as three lives may be saved. According to Government Canada, up to 52% of Canadians have reported that they or a family member have needed blood or blood products at some point in their lifetime. Many donors agree that the feeling that comes from knowing you have saved lives is well worth the short amount of time it takes to make a blood donation. Nonetheless, only a minority of potential donors actually donate blood. There are many myths about blood donation that may help explain the small percentage of donors. Knowing the facts may reaffirm your decision to donate if you are already a donor — and if you aren’t a donor already, getting the facts may help you decide to become one.
| Myth | Reality |
|---|---|
| “Your blood might become contaminated with an infection during the donation.” | There is no risk of contamination because only single-use, disposable catheters, tubing, and other equipment are used to collect blood for a donation. |
| “You are too old (or too young) to donate blood.” | There is no upper age limit on donating blood, as long as you are healthy. The minimum age is 16 years. |
| “You can’t donate blood if you have high blood pressure.” | As long as your blood pressure is below 180/100 at the time of donation, you can give blood. Even if you take blood pressure medication to keep your blood pressure below this level, you can donate. |
| “You can’t give blood if you have high cholesterol.” | Having high cholesterol does not affect your ability to donate blood. Taking cholesterol-lowering medication also does not disqualify you. |
| “You can’t donate blood if you have had a flu shot.” | Having a flu shot has no effect on your ability to donate blood. You can even donate on the same day that you receive a flu shot. |
| “You can’t donate blood if you take medication.” | As long as you are healthy, in most cases, taking medication does not preclude you from donating blood. |
| “Your blood isn’t needed if it’s a common blood type.” | All types of blood are in constant demand. |
14.5 Summary
- Blood is a fluid connective tissue that circulates throughout the body in the cardiovascular system. Blood supplies tissues with oxygen and nutrients and removes their metabolic wastes. Blood helps defend the body from pathogens and other threats, transports hormones and other substances, and helps keep the body’s pH and temperature in homeostasis.
- Plasma is the liquid component of blood, and it makes up more than half of blood by volume. It consists of water and many dissolved substances. It also contains blood cells, including erythrocytes, leukocytes and thrombocytes.
- Erythrocytes, (also known as red blood cells) are the most numerous cells in blood. They consist mostly of hemoglobin, which carries oxygen. Erythrocytes also carry antigens that determine blood type.
- Leukocytes (also referred to as white blood cells) are less numerous than erythrocytes and are part of the body’s immune system. There are several different types of leukocytes that differ in their specific immune functions. They protect the body from abnormal cells, microorganisms, and other harmful substances.
- Thrombocytes (also called platelets) are cell fragments that play important roles in blood clotting, or coagulation. They stick together at breaks in blood vessels to form a clot and stimulate the production of fibrin, which strengthens the clot.
- All blood cells form by proliferation of stem cells in red bone marrow in a process called hematopoiesis. When blood cells die, they are phagocytized by leukocytes and removed from the circulation.
- Disorders of the blood include leukemia, which is cancer of the bone-forming cells; hemophilia, which is any of several genetic blood-clotting disorders; carbon monoxide poisoning, which prevents erythrocytes from binding with oxygen and causes suffocation; HIV infection, which destroys certain types of leukocytes and can cause AIDS; and anemia, in which there are not enough erythrocytes to carry adequate oxygen to body tissues.
14.5 Review Questions
- What is blood? Why is blood considered a connective tissue?
- Identify four physiological roles of blood in the body.
- Describe plasma and its components.
-
14.5 Explore More
Joe Landolina: This gel can make you stop bleeding instantly, TED, 2014.
Can Synthetic Blood Help The World’s Blood Shortage? Science Plus, 2016.
How bones make blood – Melody Smith, TED-Ed, 2020.
Attributions
Figure 14.5.1
vampire_PNG32 from pngimg.com is used under a CC BY-NC 4.0 (https://creativecommons.org/licenses/by-nc/4.0/) license.
Figure 14.5.2
Blood-centrifugation-scheme by KnuteKnudsen at English Wikipedia on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 14.5.3
SEM_blood_cells by Bruce Wetzel and Harry Schaefer (Photographers)/ NCI AV-8202-3656 on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/en:Public_domain).
Figure 14.5.4
ABO_blood_type.svg by InvictaHOG on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/en:Public_domain).
Figure 14.5.5
Blood_clot_in_scanning_electron_microscopy by Janice Carr from CDC/ Public Health Image LIbrary (PHIL) ID #7308 on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/en:Public_domain).
Figure 14.5.6
Blausen_0740_Platelets by BruceBlaus on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 14.5.7
Platelet_Party_900x by Awkward Yeti (used with permission of the author) © All Rights Reserved
Figure 14.5.8
Symptoms_of_anemia.svg by Mikael Häggström on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/en:public_domain).
References
Blausen.com Staff. (2014). Medical gallery of Blausen Medical 2014. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436.
Blood, organ and tissue donation. (2020, April 28). Government of Canada. https://www.canada.ca/en/public-health/services/healthy-living/blood-organ-tissue-donation.html#a3
Canadian Blood Services. (n.d.). There is an immediate need for blood as demand is rising. https://www.blood.ca
Science Plus. (2016, March 2). Can synthetic blood help the world’s blood shortage? https://www.youtube.com/watch?v=hgp8LtwFSBA&feature=youtu.be
TED. (2014, November 20). Joe Landolina: This gel can make you stop bleeding instantly. YouTube. https://www.youtube.com/watch?v=e-5wqwp64MM&feature=youtu.be
TED-Ed. (2020, January 27). How bones make blood – Melody Smith. YouTube. https://www.youtube.com/watch?v=1Qfmkd6C8u8&feature=youtu.be
Structures containing neuronal cell bodies in the peripheral nervous system.
Refers to the body system consisting of the heart, blood vessels and the blood. Blood contains oxygen and other nutrients which your body needs to survive. The body takes these essential nutrients from the blood.
The smallest unit of life, consisting of at least a membrane, cytoplasm, and genetic material.
The central nervous system organ inside the skull that is the control center of the nervous system.
The process of producing cellular energy involving oxygen. Cells break down food in the mitochondria in a long, multi-step process that produces roughly 36 ATP. The first step in is glycolysis, the second is the Krebs cycle and the third is the electron transport system.
Glucose (also called dextrose) is a simple sugar with the molecular formula C6H12O6. Glucose is the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight.
Amino acids are organic compounds that combine to form proteins.
A microorganism which causes disease.
A hormone is a signaling molecule produced by glands in multicellular organisms that target distant organs to regulate physiology and behavior.
What Are You Made of?

Your entire body is made of cells and cells are made of molecules.If you look at your hand, what do you see? Of course, you see skin, which consists of cells. But what are skin cells made of? Like all living cells, they are made of matter. In fact, all things are made of matter. Matter is anything that takes up space and has mass. Matter, in turn, is made up of chemical substances. A chemical substance is matter that has a definite composition that is consistent throughout. A chemical substance may be either an element or a compound.
Elements and Atoms
An element is a pure substance. It cannot be broken down into other types of substances. Each element is made up of just one type of atom.
Structure of an Atom
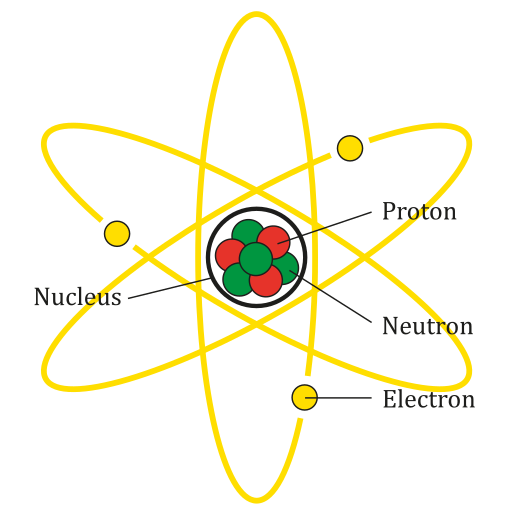
An atom is the smallest particle of an element that still has the properties of that element. Every substance is composed of atoms. Atoms are extremely small, typically about a ten-billionth of a metre in diametre. However, atoms do not have well-defined boundaries, as suggested by the atomic model shown below.
Every atom is composed of a central area — called the nucleus — and one or more subatomic particles called electrons, which move around the nucleus. The nucleus also consists of subatomic particles. It contains one or more protons and typically a similar number of neutrons. The number of protons in the nucleus determines the type of element an atom represents. An atom of hydrogen, for example, contains just one proton. Atoms of the same element may have different numbers of neutrons in the nucleus. Atoms of the same element with the same number of protons — but different numbers of neutrons — are called isotopes.
Protons have a positive electric charge and neutrons have no electric charge. Virtually all of an atom's mass is in the protons and neutrons in the nucleus. Electrons surrounding the nucleus have almost no mass, as well as a negative electric charge. If the number of protons and electrons in an atom are equal, then an atom is electrically neutral, because the positive and negative charges cancel each other out. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion.
The negatively-charged electrons of an atom are attracted to the positively-charged protons in the nucleus by a force called electromagnetic force, for which opposite charges attract. Electromagnetic force between protons in the nucleus causes these subatomic particles to repel each other, because they have the same charge. However, the protons and neutrons in the nucleus are attracted to each other by a different force, called nuclear force, which is usually stronger than the electromagnetic force. Nuclear force repels the positively-charged protons from each other.
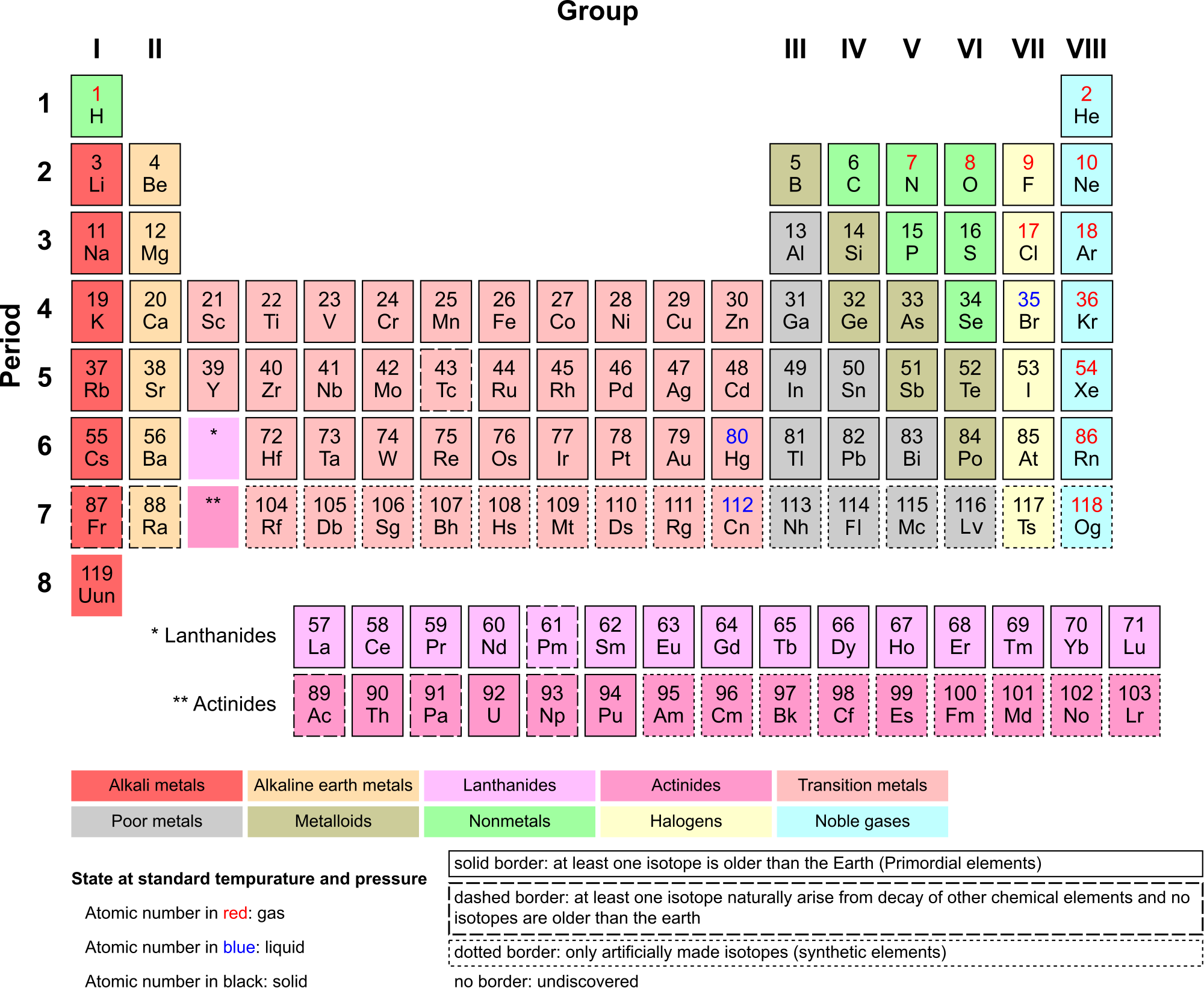
Periodic Table of the Elements
There are almost 120 known elements. As you can see in the Periodic Table of the Elements shown below, the majority of elements are metals. Examples of metals are iron (Fe) and copper (Cu). Metals are shiny and good conductors of electricity and heat. Nonmetal elements are far fewer in number. They include hydrogen (H) and oxygen (O). They lack the properties of metals.
The periodic table of the elements arranges elements in groups based on their properties. The element most important to life is carbon (C). Find carbon in the table. What type of element is it: metal or nonmetal?
Compounds and Molecules
A compound is a unique substance that consists of two or more elements combined in fixed proportions. This means that the composition of a compound is always the same. The smallest particle of most compounds in living things is called a molecule.
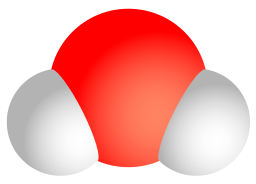
Consider water as an example. A molecule of water always contains one atom of oxygen and two atoms of hydrogen. The composition of water is expressed by the chemical formula H2O. A model of a water molecule is shown in Figure 3.2.4.
What causes the atoms of a water molecule to “stick” together? The answer is chemical bonds. A chemical bond is a force that holds together the atoms of molecules. Bonds in molecules involve the sharing of electrons among atoms. New chemical bonds form when substances react with one another. A chemical reaction is a process that changes some chemical substances into others. A chemical reaction is needed to form a compound, and another chemical reaction is needed to separate the substances in that compound.
3.2 Summary
- All matter consists of chemical substances. A chemical substance has a definite composition which is consistent throughout. A chemical substance may be either an element or a compound.
- An element is a pure substance that cannot be broken down into other types of substances.
- An atom is the smallest particle of an element that still has the properties of that element. Atoms, in turn, are composed of subatomic particles, including negative electrons, positive protons, and neutral neutrons. The number of protons in an atom determines the element it represents.
- Atoms have equal numbers of electrons and protons, so they have no charge. Ions are atoms that have lost or gained electrons, and as a result have either a positive or negative charge. Atoms with the same number of protons — but different numbers of neutrons — are called isotopes.
- There are almost 120 known elements. The majority of elements are metals. A smaller number are nonmetals. The latter include carbon, hydrogen, and oxygen.
- A compound is a substance that consists of two or more elements in a unique composition. The smallest particle of a compound is called a molecule. Chemical bonds hold together the atoms of molecules. Compounds can form only in chemical reactions, and they can break down only in other chemical reactions.
3.2 Review Questions
-
- What is an element? Give three examples.
- Define compound. Explain how compounds form.
- Compare and contrast atoms and molecules.
- The compound called water can be broken down into its constituent elements by applying an electric current to it. What ratio of elements is produced in this process?
- Relate ions and isotopes to elements and atoms.
- What is the most important element to life?
- Iron oxide is often known as rust — the reddish substance you might find on corroded metal. The chemical formula for this type of iron oxide is Fe2O3. Answer the following questions about iron oxide and briefly explain each answer.
- Is iron oxide an element or a compound?
- Would one particle of iron oxide be considered a molecule or an atom?
- Describe the relative proportion of atoms in iron oxide.
- What causes the Fe and O to stick together in iron oxide?
- Is iron oxide made of metal atoms, metalloid atoms, nonmetal atoms, or a combination of any of these?
- 14C is an isotope of carbon used in the radiocarbon dating of organic material. The most common isotope of carbon is 12C. Do you think 14C and 12C have different numbers of neutrons or protons? Explain your answer.
- Explain why ions have a positive or negative charge.
- Name the three subatomic particles described in this section.
3.2 Explore More
https://www.youtube.com/watch?v=yQP4UJhNn0I&feature=emb_logo
Just how small is an atom? TED-Ed, 2012
Attributions
Figure 3.2.1
Man Sitting, by Gregory Culmer, on Unsplash, is used under the Unsplash license (https://unsplash.com/license).
Figure 3.2.2
Lithium Atom diagram, by AG Caesar, is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0/deed.en)
Figure 3.2.3
Periodic Table Armtuk3, by Armtuk, is used under a CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/) license.
Figure 3.2.4
Water molecule, by Sakurambo, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
References
TED-Ed. (2012, April 16). Just how small is an atom. YouTube. https://www.youtube.com/watch?v=yQP4UJhNn0I&feature=youtu.be
The transfer of genetic variation from one population to another. If the rate of gene flow is high enough, then two populations are considered to have equivalent allele frequencies and therefore effectively be a single population.
Created by: CK-12/Adapted by Christine Miller

Bring on the S'mores!
This inviting camp fire can be used for both heat and light. Heat and light are two forms of energy that are released when a fuel like wood is burned. The cells of living things also get energy by "burning." They "burn" glucose in a process called cellular respiration.
What Is Cellular Respiration?
Cellular respiration is the process by which living cells break down glucose molecules and release energy. The process is similar to burning, although it doesn’t produce light or intense heat as a campfire does. This is because cellular respiration releases the energy in glucose slowly and in many small steps. It uses the energy released to form molecules of ATP, the energy-carrying molecules that cells use to power biochemical processes. In this way, cellular respiration is an example of energy coupling: glucose is broken down in an exothermic reaction, and then the energy from this reaction powers the endothermic reaction of the formation of ATP. Cellular respiration involves many chemical reactions, but they can all be summed up with this chemical equation:
C6H12O6 6O2 → 6CO2 6H2O Chemical Energy (in ATP)
In words, the equation shows that glucose (C6H12O6) and oxygen (O2) react to form carbon dioxide (CO2) and water (H2O), releasing energy in the process. Because oxygen is required for cellular respiration, it is an aerobic process.
Cellular respiration occurs in the cells of all living things, both autotrophs and heterotrophs. All of them burn glucose to form ATP. The reactions of cellular respiration can be grouped into three stages: glycolysis, the Krebs cycle (also called the citric acid cycle), and electron transport. Figure 4.10.2 gives an overview of these three stages, which are also described in detail below.
Cellular Respiration Stage I: Glycolysis
The first stage of cellular respiration is glycolysis, which happens in the cytosol of the cytoplasm.
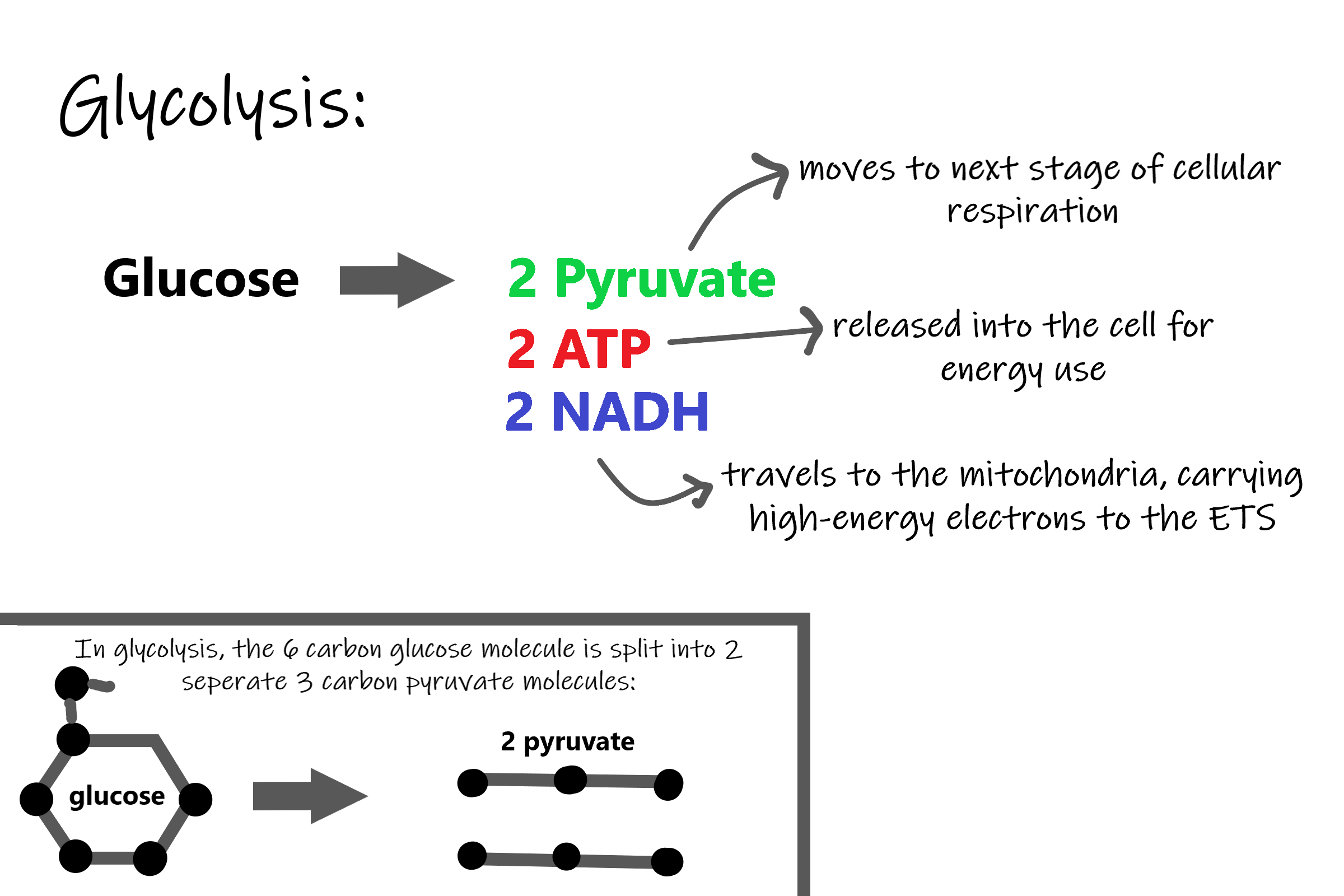
Splitting Glucose
The word glycolysis literally means “glucose splitting,” which is exactly what happens in this stage. Enzymes split a molecule of glucose into two molecules of pyruvate (also known as pyruvic acid). This occurs in several steps, as summarized in the following diagram.
Results of Glycolysis
Energy is needed at the start of glycolysis to split the glucose molecule into two pyruvate molecules which go on to stage II of cellular respiration. The energy needed to split glucose is provided by two molecules of ATP; this is called the energy investment phase. As glycolysis proceeds, energy is released, and the energy is used to make four molecules of ATP; this is the energy harvesting phase. As a result, there is a net gain of two ATP molecules during glycolysis. During this stage, high-energy electrons are also transferred to molecules of NAD to produce two molecules of NADH, another energy-carrying molecule. NADH is used in stage III of cellular respiration to make more ATP.
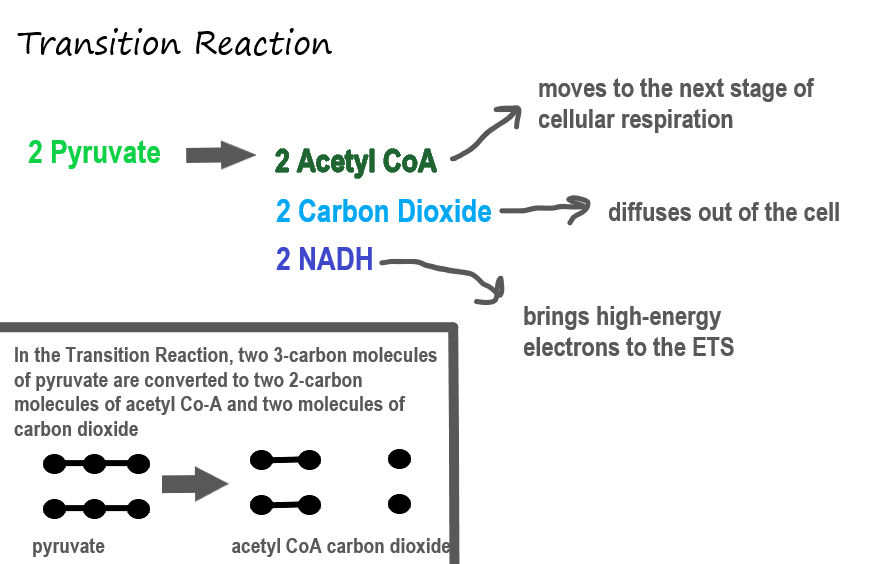
Transition Reaction
Before pyruvate can enter the next stage of cellular respiration it needs to be modified slightly. The transition reaction is a very short reaction which converts the two molecules of pyruvate to two molecules of acetyl CoA, carbon dioxide, and two high energy electron pairs convert NAD to NADH. The carbon dioxide is released, the acetyl CoA moves to the mitochondria to enter the Kreb's Cycle (stage II), and the NADH carries the high energy electrons to the Electron Transport System (stage III).
Structure of the Mitochondrion
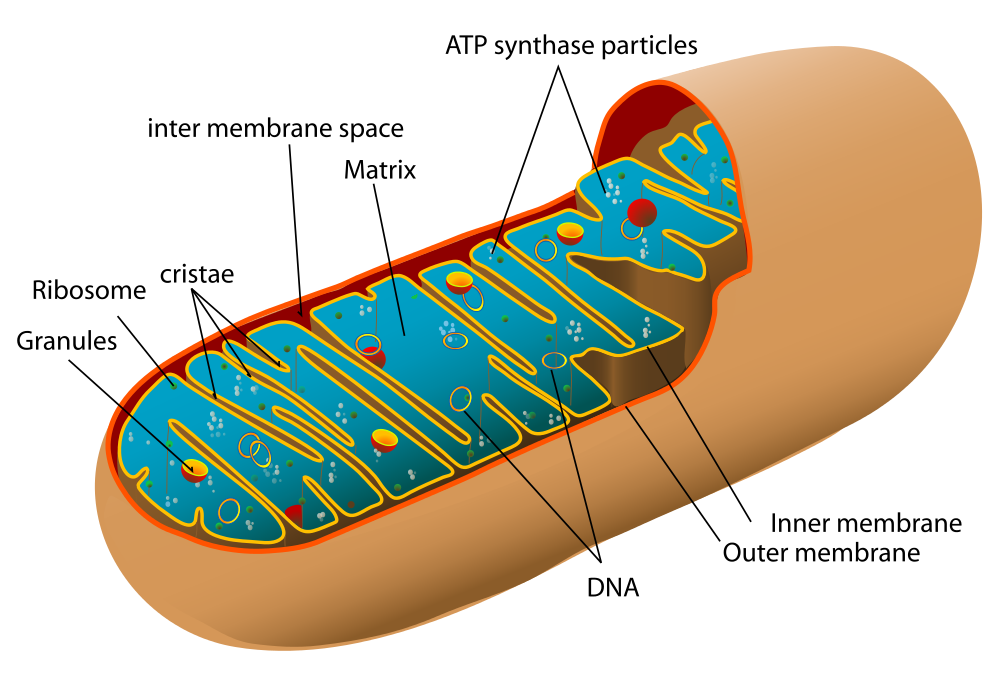
Before you read about the last two stages of cellular respiration, you need to know more about the mitochondrion, where these two stages take place. A diagram of a mitochondrion is shown in Figure 4.10.5.
The structure of a mitochondrion is defined by an inner and outer membrane. This structure plays an important role in aerobic respiration.
As you can see from the figure, a mitochondrion has an inner and outer membrane. The space between the inner and outer membrane is called the intermembrane space. The space enclosed by the inner membrane is called the matrix. The second stage of cellular respiration (the Krebs cycle) takes place in the matrix. The third stage (electron transport) happens on the inner membrane.
Cellular Respiration Stage II: The Krebs Cycle
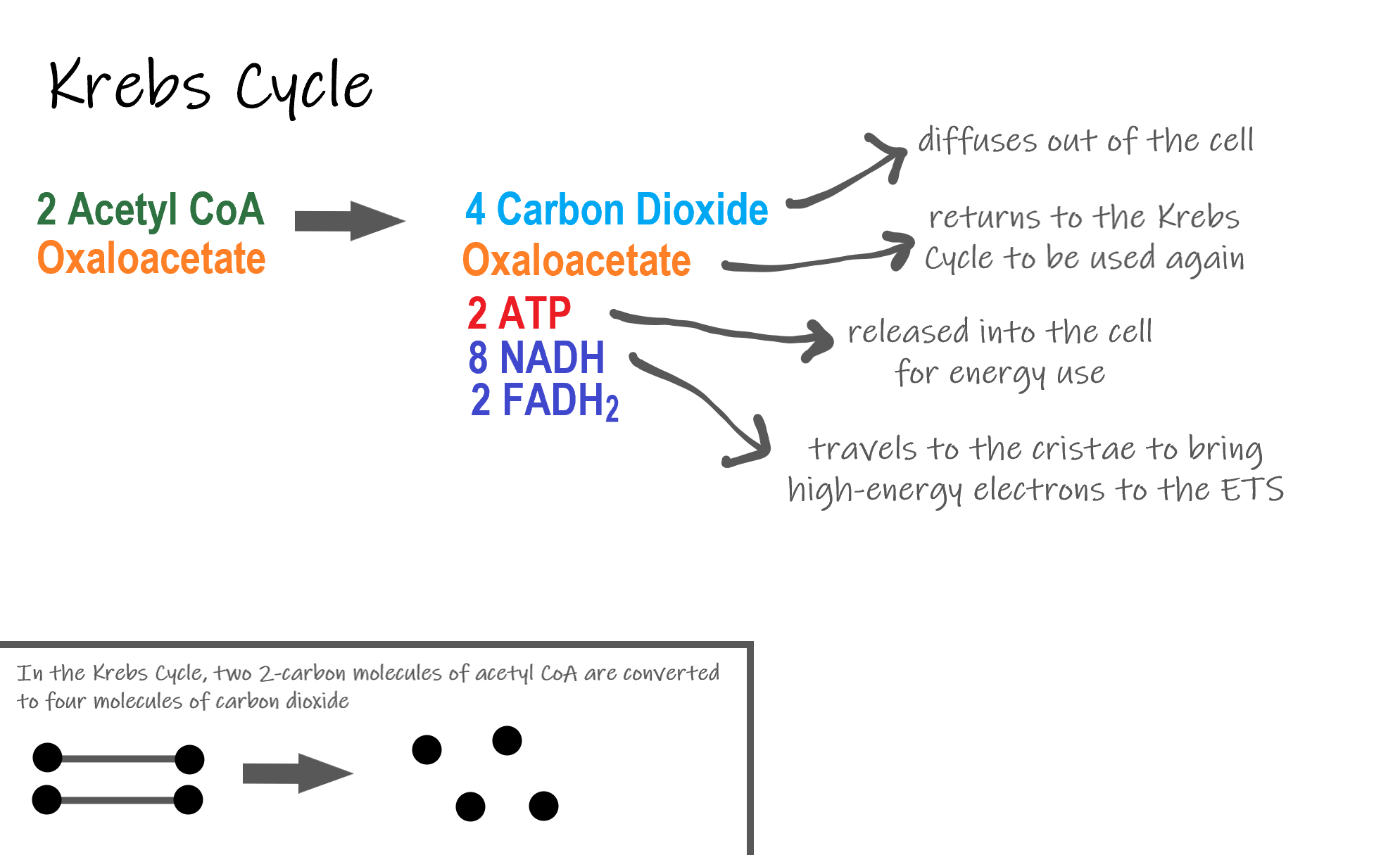
Recall that glycolysis produces two molecules of pyruvate (pyruvic acid), which are then converted to acetyl CoA during the short transition reaction. These molecules enter the matrix of a mitochondrion, where they start the Krebs cycle (also known as the Citric Acid Cycle). The reason this stage is considered a cycle is because a molecule called oxaloacetate is present at both the beginning and end of this reaction and is used to break down the two molecules of acetyl CoA. The reactions that occur next are shown in Figure 4.10.6.
Steps of the Krebs Cycle
The Krebs cycle itself actually begins when acetyl-CoA combines with a four-carbon molecule called OAA (oxaloacetate) (see Figure 4.10.6). This produces citric acid, which has six carbon atoms. This is why the Krebs cycle is also called the citric acid cycle.
After citric acid forms, it goes through a series of reactions that release energy. The energy is captured in molecules of NADH, ATP, and FADH2, another energy-carrying coenzyme. Carbon dioxide is also released as a waste product of these reactions.
The final step of the Krebs cycle regenerates OAA, the molecule that began the Krebs cycle. This molecule is needed for the next turn through the cycle. Two turns are needed because glycolysis produces two pyruvic acid molecules when it splits glucose.
Results of the Glycolysis, Transition Reaction and Krebs Cycle
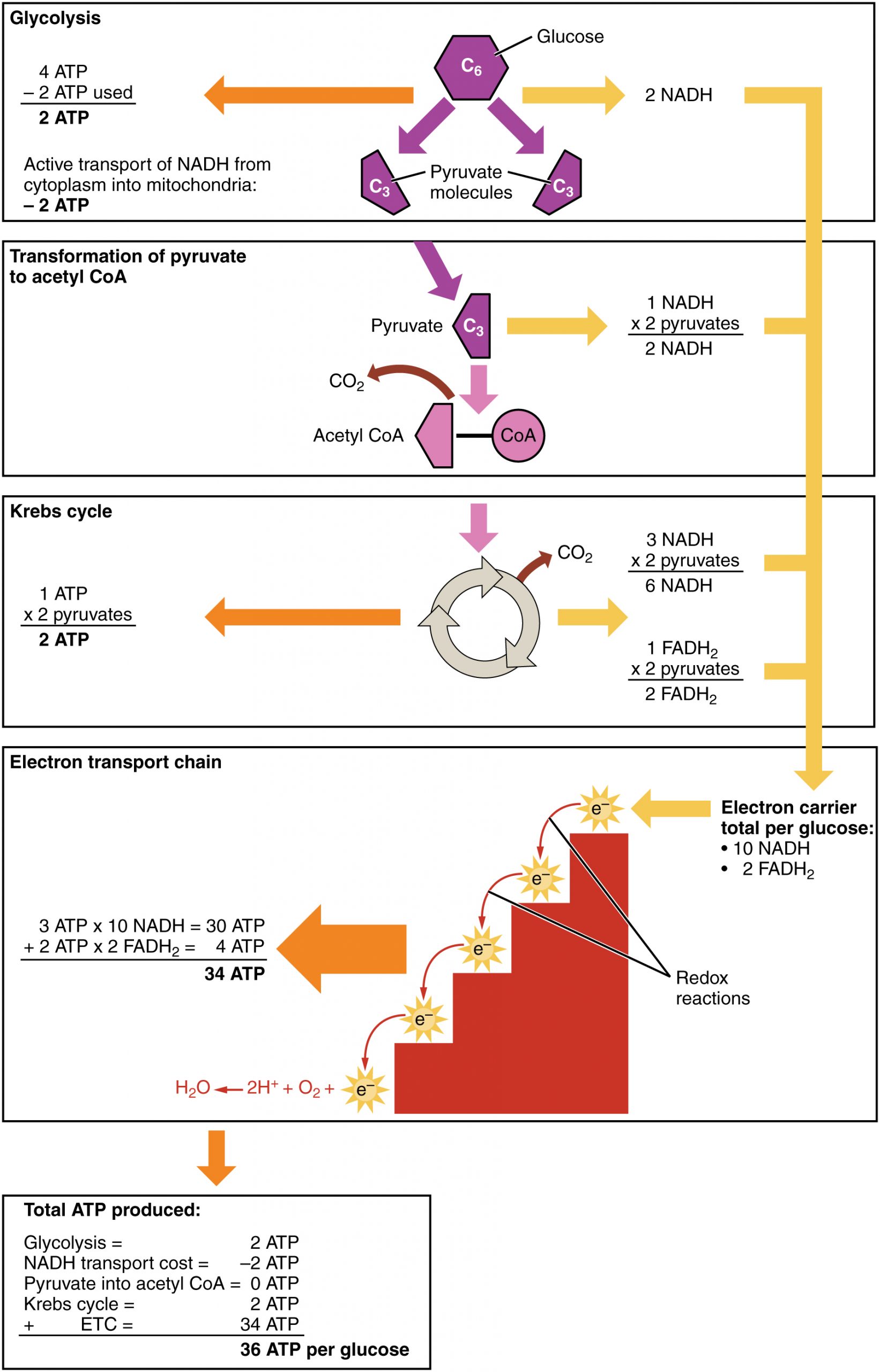
After glycolysis, transition reaction, and the Krebs cycle, the glucose molecule has been broken down completely. All six of its carbon atoms have combined with oxygen to form carbon dioxide. The energy from its chemical bonds has been stored in a total of 16 energy-carrier molecules. These molecules are:
- 4 ATP (2 from glycolysis, 2 from Krebs Cycle)
- 12 NADH (2 from glycolysis, 2 from transition reaction, and 8 from Krebs cycle)
- 2 FADH2 (both from the Krebs cycle)
The events of cellular respiration up to this point are exergonic reactions- they are releasing energy that had been stored in the bonds of the glucose molecule. This energy will be transferred to the third and final stage of cellular respiration: the Electron Transport System, which is an endergonic reaction. Using an exothermic reaction to power an endothermic reaction is known as energy coupling.
Cellular Respiration Stage III: Electron Transport Chain
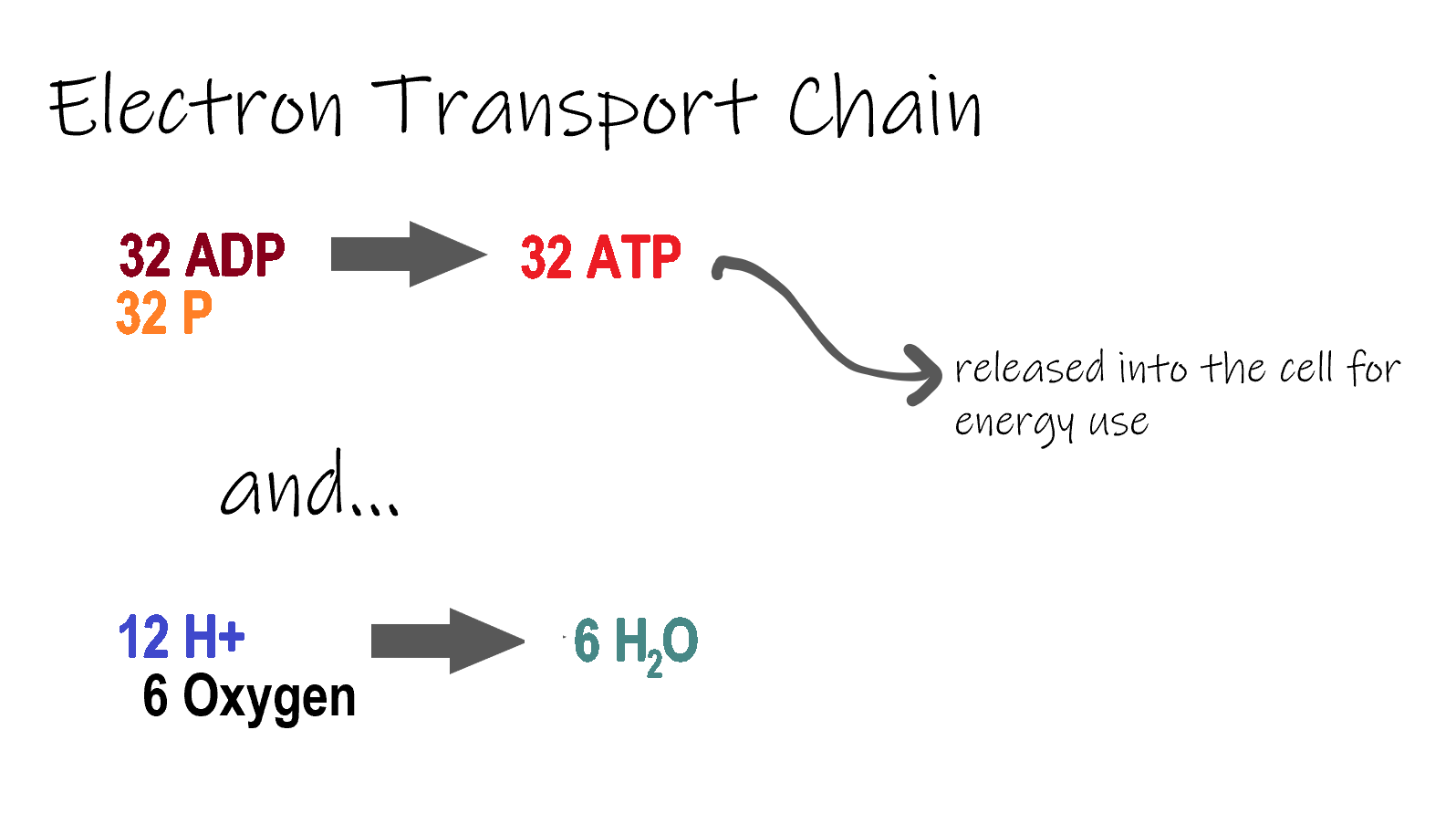
ETC, the final stage in cellular respiration produces 32 ATP. The Electron Transport Chain is the final stage of cellular respiration. In this stage, energy being transported by NADH and FADH2 is transferred to ATP. In addition, oxygen acts as the final proton acceptor for the hydrogens released from all the NADH and FADH2, forming water. Figure 4.10.8 shows the reactants and products of the ETC.
Transporting Electrons
The Electron transport chain is the third stage of cellular respiration and is illustrated in Figure 4.10.8. During this stage, high-energy electrons are released from NADH and FADH2, and they move along electron-transport chains on the inner membrane of the mitochondrion. An electron-transport chain is a series of molecules that transfer electrons from molecule to molecule by chemical reactions. Some of the energy from the electrons is used to pump hydrogen ions (H ) across the inner membrane, from the matrix into the intermembrane space. This ion transfer creates an electrochemical gradient that drives the synthesis of ATP.
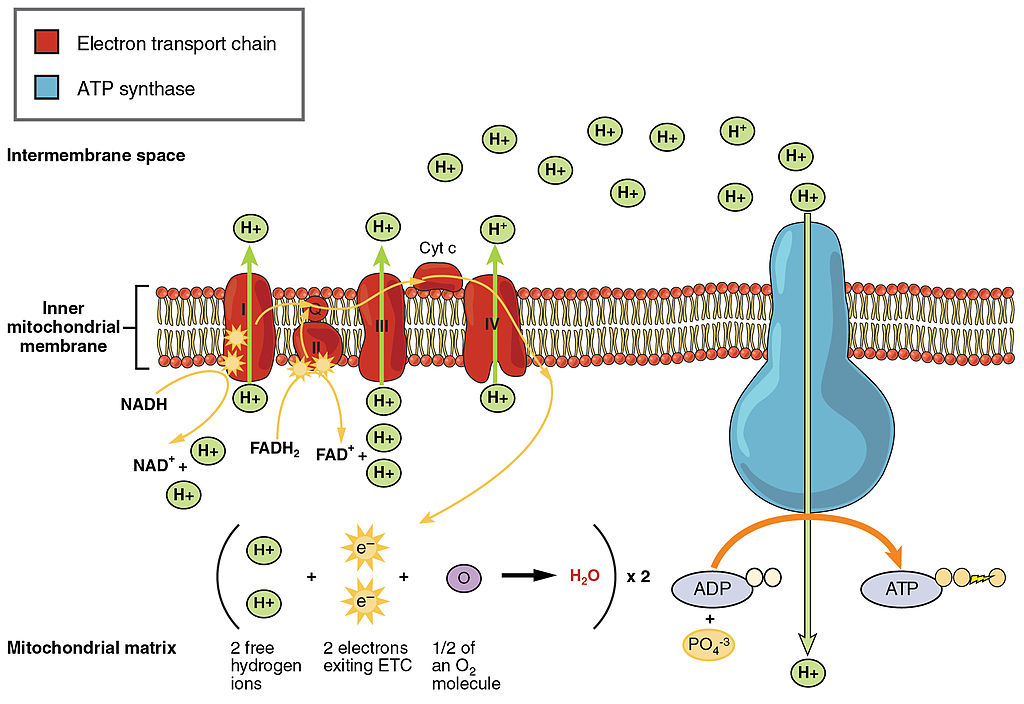
Making ATP
As shown in Figure 4.10.8, the pumping of hydrogen ions across the inner membrane creates a greater concentration of the ions in the intermembrane space than in the matrix. This gradient causes the ions to flow back across the membrane into the matrix, where their concentration is lower. ATP synthase acts as a channel protein, helping the hydrogen ions cross the membrane. It also acts as an enzyme, forming ATP from ADP and inorganic phosphate in a process called oxidative phosphorylation. After passing through the electron-transport chain, the “spent” electrons combine with oxygen to form water.
How Much ATP?
You have seen how the three stages of aerobic respiration use the energy in glucose to make ATP. How much ATP is produced in all three stages combined? Glycolysis produces two ATP molecules, and the Krebs cycle produces two more. Electron transport begins with several molecules of NADH and FADH2 from the Krebs cycle and transfers their energy into as many as 34 more ATP molecules. All told, then, up to 38 molecules of ATP can be produced from just one molecule of glucose in the process of cellular respiration.
4.10 Summary
- Cellular respiration is the aerobic process by which living cells break down glucose molecules, release energy, and form molecules of ATP. Generally speaking, this three-stage process involves glucose and oxygen reacting to form carbon dioxide and water.
- The first stage of cellular respiration, called glycolysis, takes place in the cytoplasm. In this step, enzymes split a molecule of glucose into two molecules of pyruvate, which releases energy that is transferred to ATP. Following glycolysis, a short reaction called the transition reaction converts the pyruvate into two molecules of acetyl CoA.
- The organelle called a mitochondrion is the site of the other two stages of cellular respiration. The mitochondrion has an inner and outer membrane separated by an intermembrane space, and the inner membrane encloses a space called the matrix.
- The second stage of cellular respiration, called the Krebs cycle, takes place in the matrix of a mitochondrion. During this stage, two turns through the cycle result in all of the carbon atoms from the two pyruvate molecules forming carbon dioxide and the energy from their chemical bonds being stored in a total of 16 energy-carrying molecules (including two from glycolysis and two from transition reaction).
- The third and final stage of cellular respiration, called electron transport, takes place on the inner membrane of the mitochondrion. Electrons are transported from molecule to molecule down an electron-transport chain. Some of the energy from the electrons is used to pump hydrogen ions across the membrane, creating an electrochemical gradient that drives the synthesis of many more molecules of ATP.
- In all three stages of cellular respiration combined, as many as 38 molecules of ATP are produced from just one molecule of glucose.
4.10 Review Questions
- What is the purpose of cellular respiration? Provide a concise summary of the process.
- State what happens during glycolysis.
- Describe the structure of a mitochondrion.
- What molecule is present at both the beginning and end of the Krebs cycle?
- What happens during the electron transport stage of cellular respiration?
- How many molecules of ATP can be produced from one molecule of glucose during all three stages of cellular respiration combined?
- Do plants undergo cellular respiration? Why or why not?
- Explain why the process of cellular respiration described in this section is considered aerobic.
- Name three energy-carrying molecules involved in cellular respiration.
-
- Which stage of aerobic cellular respiration produces the most ATP?
-
4.10 Explore More
https://www.youtube.com/watch?time_continue=2&v=00jbG_cfGuQ&feature=emb_logo
ATP & Respiration: Crash Course Biology #7, CrashCourse, 2012.
https://www.youtube.com/watch?v=4Eo7JtRA7lg&t=3s
Cellular Respiration and the Mighty Mitochondria, The Amoeba Sisters, 2014.
Attributions
Figure 4.10.1
Smores by Jessica Ruscello on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 4.10.2
Carbohydrate_Metabolism by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 4.10.3
Glycolysis by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.4
Transition Reaction by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.5
Mitochondrion by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.10.6
Krebs cycle by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.7
Electron Transport Chain (ETC) by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Figure 4.10.8
The_Electron_Transport_Chain by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
References
CrashCourse. (2012, March 12). ATP & Respiration: Crash Course Biology #7. YouTube. https://www.youtube.com/watch?time_continue=2&v=00jbG_cfGuQ&feature=emb_logo
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 24.8 Electron Transport Chain [digital image]. In Anatomy & Physiology, Connexions (Section ). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/24-2-carbohydrate-metabolism
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 24.9 Carbohydrate Metabolism [digital image]. In Anatomy & Physiology, Connexions (Section 24.2). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/24-2-carbohydrate-metabolism
The Amoeba Sisters. (2014, October 22). Cellular Respiration and the Mighty Mitochondria. YouTube. https://www.youtube.com/watch?v=4Eo7JtRA7lg&t=3s
A class of biological molecule consisting of linked monomers of amino acids and which are the most versatile macromolecules in living systems and serve crucial functions in essentially all biological processes.

Danger! Acid!
You probably know that batteries contain dangerous chemicals, including strong acids. Strong acids can hurt you if they come into contact with your skin or eyes. Therefore, it may surprise you to learn that your life depends on acids. There are many acids inside your body, and some of them are as strong as battery acid. Acids are needed for digestion and some forms of energy production. Genes are made of nucleic acids, proteins of amino acids, and lipids of fatty acids.
Water and Solutions
Acids (such as battery acid) are solutions. A solution is a mixture of two or more substances that has the same composition throughout. Many solutions are a mixture of water and some other substance. Not all solutions are acids. Some are bases and some are neither acids nor bases. To understand acids and bases, you need to know more about pure water.
In pure water (such as distilled water), a tiny fraction of water molecules naturally breaks down to form ions. An ion is an electrically charged atom or molecule. The breakdown of water is represented by the chemical equation:
2 H2O → H3O+ + OH-
The products of this reaction are a hydronium ion (H3O+) and a hydroxide ion (OH-). The hydroxide ion, which has a negative charge, forms when a water molecule gives up a positively charged hydrogen ion (H+). The hydronium ion, which has a positive charge, forms when another water molecule accepts the hydrogen ion.
Acidity and pH
The concentration of hydronium ions in a solution is known as acidity. In pure water, the concentration of hydronium ions is very low; only about one in ten million water molecules naturally breaks down to form a hydronium ion. As a result, pure water is essentially neutral. Acidity is measured on a scale called pH, as shown in Figure 3.12.2. Pure water has a pH of 7, so the point of neutrality on the pH scale is 7.
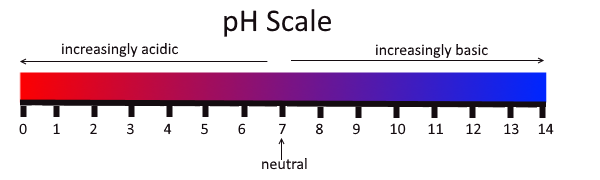
This pH scale shows the acidity of many common substances. The lower the pH value, the more acidic a substance is.

Acids
If a solution has a higher concentration of hydronium ions than pure water, it has a pH lower than 7. A solution with a pH lower than 7 is called an acid. As the hydronium ion concentration increases, the pH value decreases. Therefore, the more acidic a solution is, the lower its pH value is.
Did you ever taste vinegar? Like other acids, it tastes sour. Stronger acids can be harmful to organisms. Even stomach acid would eat through the stomach if it were not lined with a layer of mucus. Strong acids can also damage materials, even hard materials such as glass.
Bases
If a solution has a lower concentration of hydronium ions than pure water, it has a pH higher than 7. A solution with a pH higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. Like strong acids, strong bases can harm organisms and damage materials. For example, lye can burn the skin, and bleach can remove the colour from clothing.
Buffers
A buffer is a solution that can resist changes in pH. Buffers are able to maintain a certain pH by by absorbing any H+ or OH- ions added to the solution. Buffers are extremely important in biological systems in order to maintain a pH conducive to life. Bicarbonate is an example of a buffer which is used to maintain pH of the blood. In this buffering system, if blood becomes too acidic, carbonic acid will convert to carbon dioxide and water. If the blood becomes too basic, carbonic acid will convert to bicarbonate and H+ ions:
CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+
Acids, Bases, and Enzymes
Many acids and bases in living things provide the pH that enzymes need. Enzymes are biological catalysts that must work effectively for biochemical reactions to occur. Most enzymes can do their job only at a certain level of acidity. Cells secrete acids and bases to maintain the proper pH for enzymes to do their work.
Every time you digest food, acids and bases are at work in your digestive system. Consider the enzyme pepsin, which helps break down proteins in the stomach. Pepsin needs an acidic environment to do its job. The stomach secretes a strong acid called hydrochloric acid that allows pepsin to work. When stomach contents enter the small intestine, the acid must be neutralized, because enzymes in the small intestine need a basic environment in order to work. An organ called the pancreas secretes a base named bicarbonate into the small intestine, and this base neutralizes the acid.
Feature: My Human Body
Do you ever have heartburn? The answer is probably "yes." More than 60 million Americans have heartburn at least once a month, and more than 15 million suffer from it on a daily basis. Knowing more about heartburn may help you prevent it or know when it's time to seek medical treatment.
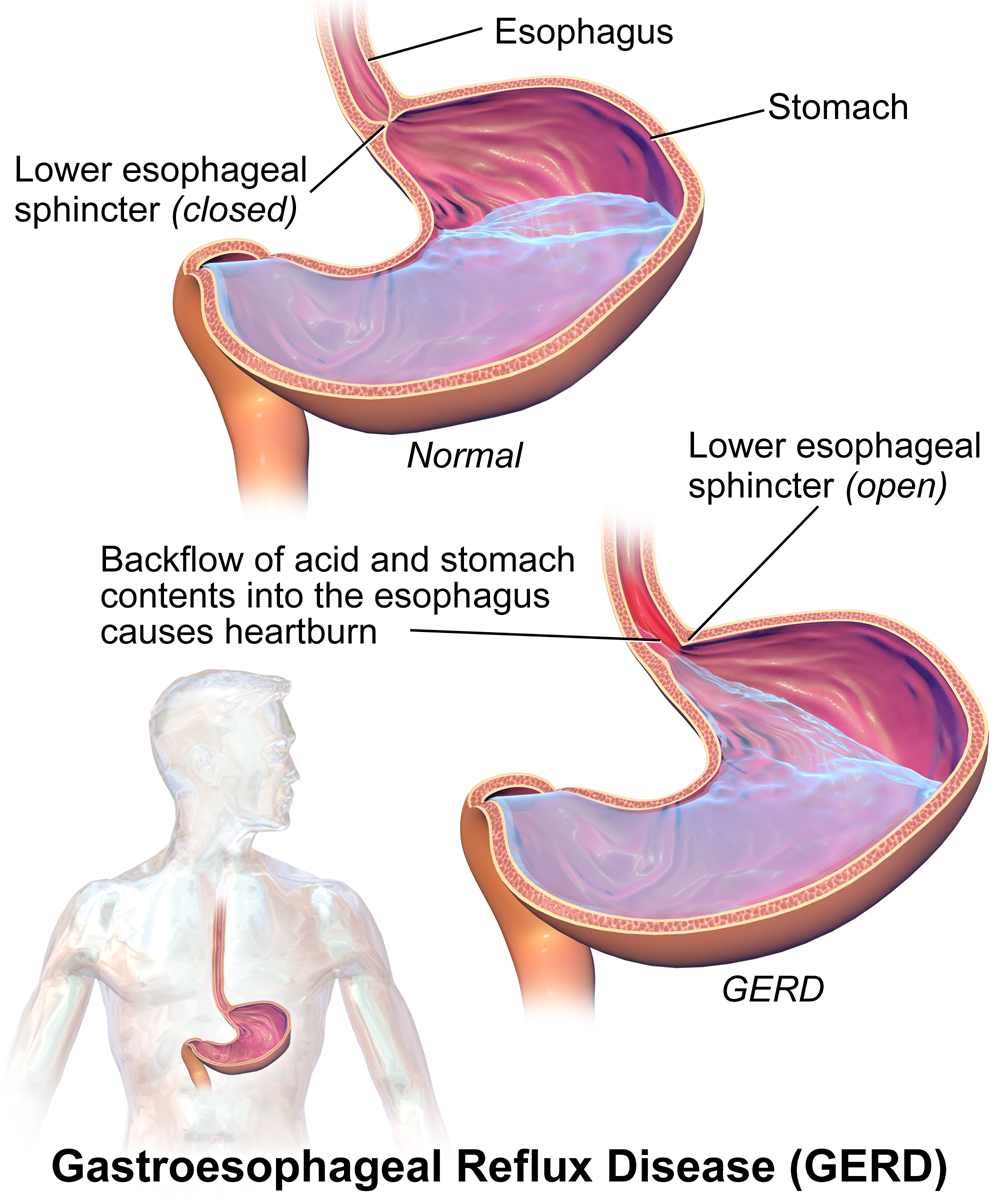
Heartburn doesn't have anything to do with the heart, but it does cause a burning sensation in the vicinity of the chest. Normally, the acid secreted into the stomach remains in the stomach where it is needed to allow pepsin to do its job of digesting proteins. A long tube called the esophagus carries food from the mouth to the stomach. A sphincter, or valve, between the esophagus and stomach opens to allow swallowed food to enter the stomach and then closes to prevent stomach contents from backflowing into the esophagus. If this sphincter is weak or relaxes inappropriately, stomach contents flow into the esophagus. Because stomach contents are usually acidic, this causes the burning sensation known as heartburn. People who are prone to heartburn and suffer from it often may be diagnosed with GERD, which stands for gastroesophageal reflux disease.
GERD — as well as occasional heartburn — often can be improved by dietary and other lifestyle changes that decrease the amount and acidity of reflux from the stomach into the esophagus.
- Some foods and beverages seem to contribute to GERD, so these should be avoided. Problematic foods include chocolate, fatty foods, peppermint, coffee, and alcoholic beverages.
- Decreasing portion size and eating the last meal of the day at least a couple of hours before bedtime may reduce the risk of reflux occurring.
- Smoking tends to weaken the lower esophageal sphincter, so quitting the habit may help control reflux.
- GERD is often associated with being overweight. Losing weight often brings improvement.
- Some people are helped by sleeping with the head of the bed elevated. This allows gravity to help control the backflow of acids into the esophagus from the stomach.
If you have frequent heartburn and lifestyle changes don't help, you may need medication to control the condition. Over-the-counter (OTC) antacids may be all that you need to control the occasional heartburn attack. OTC medications are usually bases that neutralize stomach acids. They may also create bubbles that help block stomach contents from entering the esophagus. For some people, OTC medications are not enough, and prescription medications are instead required for the control of GERD. These prescription medications generally work by inhibiting acid secretion in the stomach.
Be sure to see a doctor if you can't control your heartburn, or you have it often. Untreated GERD not only interferes with quality of life, it may also lead to more serious complications, ranging from esophageal bleeding to esophageal cancer.
3.12 Summary
- A solution is a mixture of two or more substances that has the same composition throughout. Many solutions consist of water and one or more dissolved substances.
- Acidity is a measure of the hydronium ion concentration in a solution. Pure water has a very low concentration and a pH of 7, which is the point of neutrality on the pH scale.
- Acids have a higher hydronium ion concentration than pure water and a pH lower than 7. Bases have a lower hydronium ion concentration than pure water and a pH higher than 7.
- Many acids and bases in living things are secreted to provide the proper pH for enzymes to work properly. Enzymes are the biological catalysts (like pepsin) needed to digest protein in the stomach. Pepsin requires an acidic environment.
3.12 Review Questions
-
- What is a solution?
- Define acidity.
- Explain how acidity is measured.
- Compare and contrast acids and bases.
- Hydrochloric acid is secreted by the stomach to provide an acidic environment for the enzyme pepsin. What is the pH of this acid? How strong of an acid is it compared with other acids?
- Define an ion. Identify the ions in the equation below, and explain what makes them ions:
- 2 H2O → H3O+ + OH-
- Explain why the pancreas secretes bicarbonate into the small intestine.
- Do you think pepsin would work in the small intestine? Why or why not?
- You may have mixed vinegar and baking soda and noticed that they bubble and react with each other. Explain why this happens. Explain also what happens to the pH of this solution after you mix the vinegar and baking soda.
- Pregnancy hormones can cause the lower esophageal sphincter to relax. What effect do you think this has on pregnant women? Explain your answer.
3.12 Explore More
https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
pH and Buffers by Bozeman Science, 2014.
https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton, TED-Ed, 2013.
Attributions
Figure 3.12.1
Leaky battery by Carbon Arc on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
Figure 3.12.2
PH_Scale by Christinelmiller on Wikimedia Commons is used under a © CC0 1.0 (https://creativecommons.org/publicdomain/zero/1.0/) public domain dedication license.
Figure 3.12.3
Ph scale with examples by OpenStax College, on Wikimedia Commons, is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 3.12.4
GERD by BruceBlaus on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
References
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 26.15 The pH Scale [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/26-4-acid-base-balance
Bozeman Science. (2014, February 22). pH and buffers. YouTube. https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
TED-Ed. (2013, October 24). The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
a colorless cell that circulates in the blood and body fluids and is involved in counteracting foreign substances and disease; a white (blood) cell. There are several types, all amoeboid cells with a nucleus, including lymphocytes, granulocytes, monocytes, and macrophages.

Created by: CK-12/Adapted by Christine Miller
Danger! Acid!
You probably know that batteries contain dangerous chemicals, including strong acids. Strong acids can hurt you if they come into contact with your skin or eyes. Therefore, it may surprise you to learn that your life depends on acids. There are many acids inside your body, and some of them are as strong as battery acid. Acids are needed for digestion and some forms of energy production. Genes are made of nucleic acids, proteins of amino acids, and lipids of fatty acids.
Water and Solutions
Acids (such as battery acid) are solutions. A solution is a mixture of two or more substances that has the same composition throughout. Many solutions are a mixture of water and some other substance. Not all solutions are acids. Some are bases and some are neither acids nor bases. To understand acids and bases, you need to know more about pure water.
In pure water (such as distilled water), a tiny fraction of water molecules naturally breaks down to form ions. An ion is an electrically charged atom or molecule. The breakdown of water is represented by the chemical equation:
2 H2O → H3O+ + OH-
The products of this reaction are a hydronium ion (H3O+) and a hydroxide ion (OH-). The hydroxide ion, which has a negative charge, forms when a water molecule gives up a positively charged hydrogen ion (H+). The hydronium ion, which has a positive charge, forms when another water molecule accepts the hydrogen ion.
Acidity and pH
The concentration of hydronium ions in a solution is known as acidity. In pure water, the concentration of hydronium ions is very low; only about one in ten million water molecules naturally breaks down to form a hydronium ion. As a result, pure water is essentially neutral. Acidity is measured on a scale called pH, as shown in Figure 3.12.2. Pure water has a pH of 7, so the point of neutrality on the pH scale is 7.

This pH scale shows the acidity of many common substances. The lower the pH value, the more acidic a substance is.

Acids
If a solution has a higher concentration of hydronium ions than pure water, it has a pH lower than 7. A solution with a pH lower than 7 is called an acid. As the hydronium ion concentration increases, the pH value decreases. Therefore, the more acidic a solution is, the lower its pH value is.
Did you ever taste vinegar? Like other acids, it tastes sour. Stronger acids can be harmful to organisms. Even stomach acid would eat through the stomach if it were not lined with a layer of mucus. Strong acids can also damage materials, even hard materials such as glass.
Bases
If a solution has a lower concentration of hydronium ions than pure water, it has a pH higher than 7. A solution with a pH higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. Like strong acids, strong bases can harm organisms and damage materials. For example, lye can burn the skin, and bleach can remove the colour from clothing.
Buffers
A buffer is a solution that can resist changes in pH. Buffers are able to maintain a certain pH by by absorbing any H+ or OH- ions added to the solution. Buffers are extremely important in biological systems in order to maintain a pH conducive to life. Bicarbonate is an example of a buffer which is used to maintain pH of the blood. In this buffering system, if blood becomes too acidic, carbonic acid will convert to carbon dioxide and water. If the blood becomes too basic, carbonic acid will convert to bicarbonate and H+ ions:
CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+
Acids, Bases, and Enzymes
Many acids and bases in living things provide the pH that enzymes need. Enzymes are biological catalysts that must work effectively for biochemical reactions to occur. Most enzymes can do their job only at a certain level of acidity. Cells secrete acids and bases to maintain the proper pH for enzymes to do their work.
Every time you digest food, acids and bases are at work in your digestive system. Consider the enzyme pepsin, which helps break down proteins in the stomach. Pepsin needs an acidic environment to do its job. The stomach secretes a strong acid called hydrochloric acid that allows pepsin to work. When stomach contents enter the small intestine, the acid must be neutralized, because enzymes in the small intestine need a basic environment in order to work. An organ called the pancreas secretes a base named bicarbonate into the small intestine, and this base neutralizes the acid.
Feature: My Human Body
Do you ever have heartburn? The answer is probably "yes." More than 60 million Americans have heartburn at least once a month, and more than 15 million suffer from it on a daily basis. Knowing more about heartburn may help you prevent it or know when it's time to seek medical treatment.

Heartburn doesn't have anything to do with the heart, but it does cause a burning sensation in the vicinity of the chest. Normally, the acid secreted into the stomach remains in the stomach where it is needed to allow pepsin to do its job of digesting proteins. A long tube called the esophagus carries food from the mouth to the stomach. A sphincter, or valve, between the esophagus and stomach opens to allow swallowed food to enter the stomach and then closes to prevent stomach contents from backflowing into the esophagus. If this sphincter is weak or relaxes inappropriately, stomach contents flow into the esophagus. Because stomach contents are usually acidic, this causes the burning sensation known as heartburn. People who are prone to heartburn and suffer from it often may be diagnosed with GERD, which stands for gastroesophageal reflux disease.
GERD — as well as occasional heartburn — often can be improved by dietary and other lifestyle changes that decrease the amount and acidity of reflux from the stomach into the esophagus.
- Some foods and beverages seem to contribute to GERD, so these should be avoided. Problematic foods include chocolate, fatty foods, peppermint, coffee, and alcoholic beverages.
- Decreasing portion size and eating the last meal of the day at least a couple of hours before bedtime may reduce the risk of reflux occurring.
- Smoking tends to weaken the lower esophageal sphincter, so quitting the habit may help control reflux.
- GERD is often associated with being overweight. Losing weight often brings improvement.
- Some people are helped by sleeping with the head of the bed elevated. This allows gravity to help control the backflow of acids into the esophagus from the stomach.
If you have frequent heartburn and lifestyle changes don't help, you may need medication to control the condition. Over-the-counter (OTC) antacids may be all that you need to control the occasional heartburn attack. OTC medications are usually bases that neutralize stomach acids. They may also create bubbles that help block stomach contents from entering the esophagus. For some people, OTC medications are not enough, and prescription medications are instead required for the control of GERD. These prescription medications generally work by inhibiting acid secretion in the stomach.
Be sure to see a doctor if you can't control your heartburn, or you have it often. Untreated GERD not only interferes with quality of life, it may also lead to more serious complications, ranging from esophageal bleeding to esophageal cancer.
3.12 Summary
- A solution is a mixture of two or more substances that has the same composition throughout. Many solutions consist of water and one or more dissolved substances.
- Acidity is a measure of the hydronium ion concentration in a solution. Pure water has a very low concentration and a pH of 7, which is the point of neutrality on the pH scale.
- Acids have a higher hydronium ion concentration than pure water and a pH lower than 7. Bases have a lower hydronium ion concentration than pure water and a pH higher than 7.
- Many acids and bases in living things are secreted to provide the proper pH for enzymes to work properly. Enzymes are the biological catalysts (like pepsin) needed to digest protein in the stomach. Pepsin requires an acidic environment.
3.12 Review Questions
-
- What is a solution?
- Define acidity.
- Explain how acidity is measured.
- Compare and contrast acids and bases.
- Hydrochloric acid is secreted by the stomach to provide an acidic environment for the enzyme pepsin. What is the pH of this acid? How strong of an acid is it compared with other acids?
- Define an ion. Identify the ions in the equation below, and explain what makes them ions:
- 2 H2O → H3O+ + OH-
- Explain why the pancreas secretes bicarbonate into the small intestine.
- Do you think pepsin would work in the small intestine? Why or why not?
- You may have mixed vinegar and baking soda and noticed that they bubble and react with each other. Explain why this happens. Explain also what happens to the pH of this solution after you mix the vinegar and baking soda.
- Pregnancy hormones can cause the lower esophageal sphincter to relax. What effect do you think this has on pregnant women? Explain your answer.
3.12 Explore More
https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
pH and Buffers by Bozeman Science, 2014.
https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton, TED-Ed, 2013.
Attributions
Figure 3.12.1
Leaky battery by Carbon Arc on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
Figure 3.12.2
PH_Scale by Christinelmiller on Wikimedia Commons is used under a © CC0 1.0 (https://creativecommons.org/publicdomain/zero/1.0/) public domain dedication license.
Figure 3.12.3
Ph scale with examples by OpenStax College, on Wikimedia Commons, is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 3.12.4
GERD by BruceBlaus on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
References
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 26.15 The pH Scale [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/26-4-acid-base-balance
Bozeman Science. (2014, February 22). pH and buffers. YouTube. https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
TED-Ed. (2013, October 24). The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
A central organelle containing hereditary material.
A tiny cellular structure that performs specific functions within a cell.
Created by CK-12 Foundation/Adapted by Christine Miller

http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2019/06/human-heartbeat-daniel_simon.mp3
Lub, Dub
Lub dub, lub dub, lub dub... That’s how the sound of a beating heart is typically described. Those are also the only two sounds that should be audible when listening to a normal, healthy heart through a stethoscope, as in Figure 14.3.1. If a doctor hears something different from the normal lub dub sounds, it’s a sign of a possible heart abnormality. What causes the heart to produce the characteristic lub dub sounds? Read on to find out.
Introduction to the Heart
The heart is a muscular organ behind the sternum (breastbone), slightly to the left of the center of the chest. A normal adult heart is about the size of a fist. The function of the heart is to pump blood through blood vessels of the cardiovascular system. The continuous flow of blood through the system is necessary to provide all the cells of the body with oxygen and nutrients, and to remove their metabolic wastes.
Structure of the Heart
The heart has a thick muscular wall that consists of several layers of tissue. Internally, the heart is divided into four chambers through which blood flows. Because of heart valves, blood flows in just one direction through the chambers.
Heart Wall
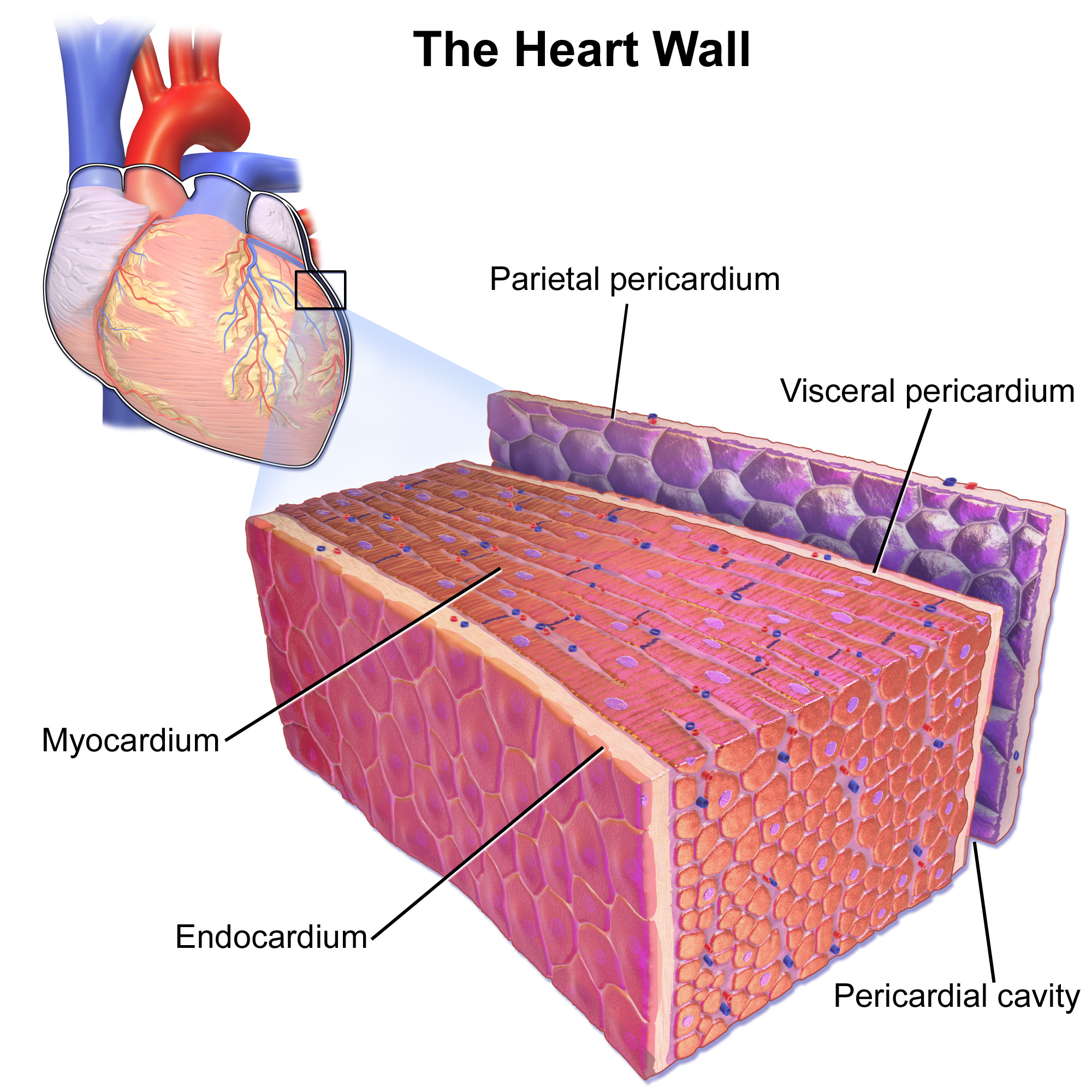
As shown in Figure 14.3.2, the wall of the heart is made up of three layers, called the endocardium, myocardium, and pericardium.
- The endocardium is the innermost layer of the heart wall. It is made up primarily of simple epithelial cells. It covers the heart chambers and valves. A thin layer of connective tissue joins the endocardium to the myocardium.
- The myocardium is the middle and thickest layer of the heart wall. It consists of cardiac muscle surrounded by a framework of collagen. There are two types of cardiac muscle cells in the myocardium: cardiomyocytes — which have the ability to contract easily — and pacemaker cells, which conduct electrical impulses that cause the cardiomyocytes to contract. About 99 per cent of cardiac muscle cells are cardiomyocytes, and the remaining one per cent is pacemaker cells. The myocardium is supplied with blood vessels and nerve fibres via the pericardium.
- The pericardium is a protective sac that encloses and protects the heart. The pericardium consists of two membranes (visceral pericardium and parietal pericardium), between which there is a fluid-filled cavity. The fluid helps to cushion the heart, and also lubricates its outer surface.
Heart Chambers
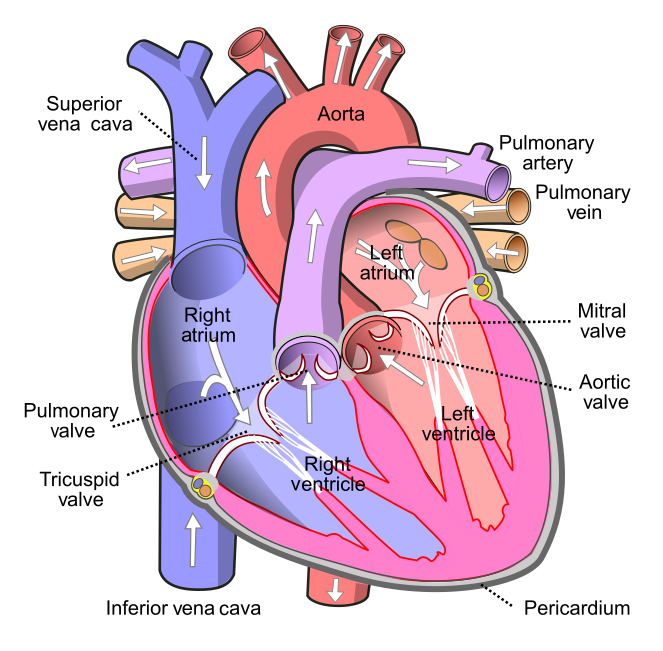
As shown in Figure 14.3.3 the four chambers of the heart include two upper chambers called atria (singular, atrium), and two lower chambers called ventricles. The atria are also referred to as receiving chambers, because blood coming into the heart first enters these two chambers. The right atrium receives deoxygenated blood from the upper and lower body through the superior vena cava and inferior vena cava, respectively. The left atrium receives oxygenated blood from the lungs through the pulmonary veins. The ventricles are also referred to as discharging chambers, because blood leaving the heart passes out through these two chambers. The right ventricle discharges blood to the lungs through the pulmonary artery, and the left ventricle discharges blood to the rest of the body through the aorta. The four chambers are separated from each other by dense connective tissue consisting mainly of collagen.
Heart Valves
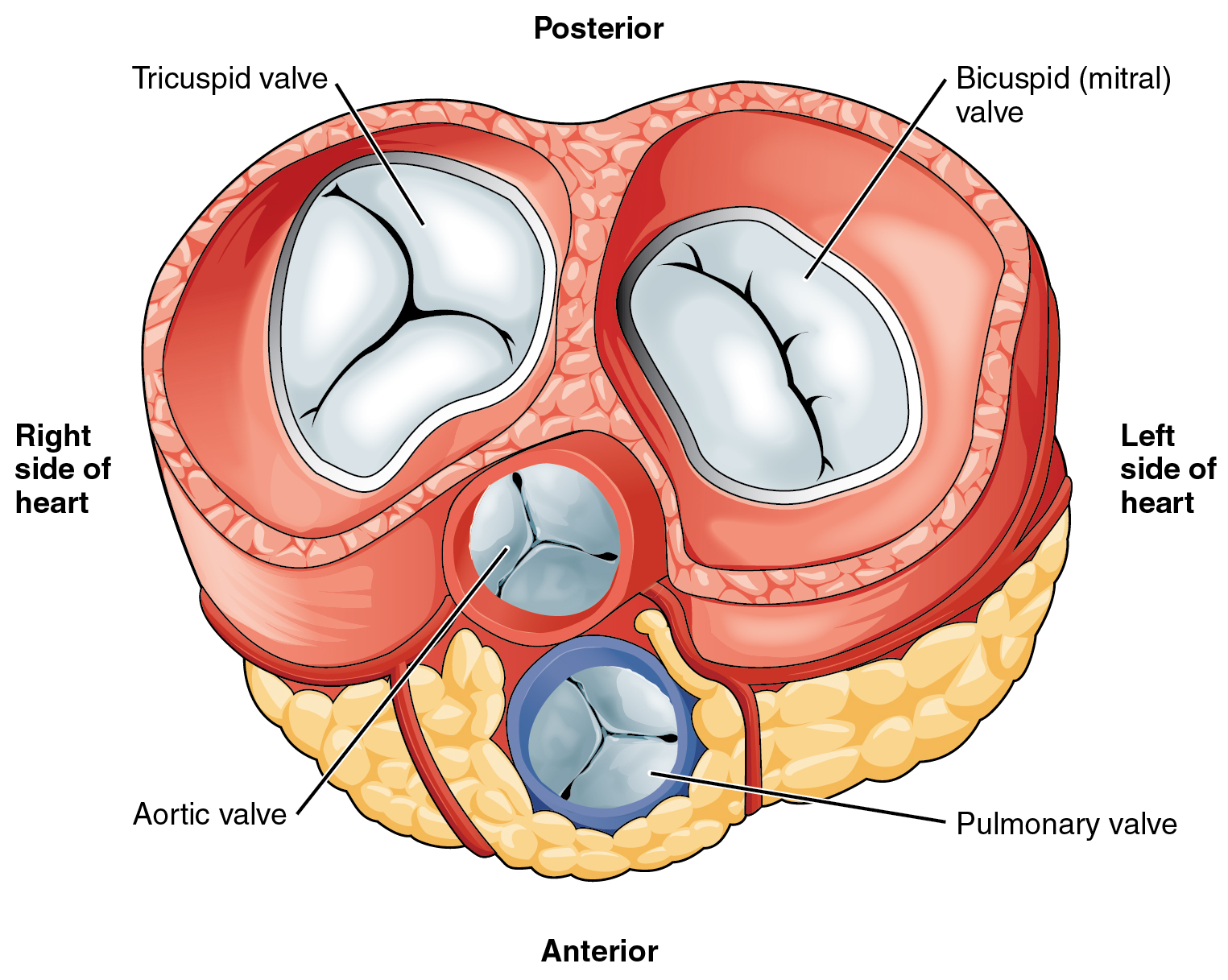
Figure 14.3.4 shows the location of the heart's four valves in a top-down view, looking down at the heart as if the arteries and veins feeding into and out of the heart were removed. The heart valves allow blood to flow from the atria to the ventricles, and from the ventricles to the pulmonary artery and aorta. The valves are constructed in such a way that blood can flow through them in only one direction, thus preventing the backflow of blood. Figure 14.3.5 shows how valves open to let blood into the appropriate chamber, and then close to prevent blood from moving in the wrong direction and the next chamber contracts. The four valves are the:
- Tricuspid atrioventricular valve, (can be shortened to tricuspid AV valve) which allows blood to flow from the right atrium to the right ventricle.
- Bicuspid atrioventricular valve (also know as the mitral valve), which allows blood to flow from the left atrium to the left ventricle.
- Pulmonary semilunar valve, which allows blood to flow from the right ventricle to the pulmonary artery.
- Aortic semilunar valve, which allows blood to flow from the left ventricle to the aorta.
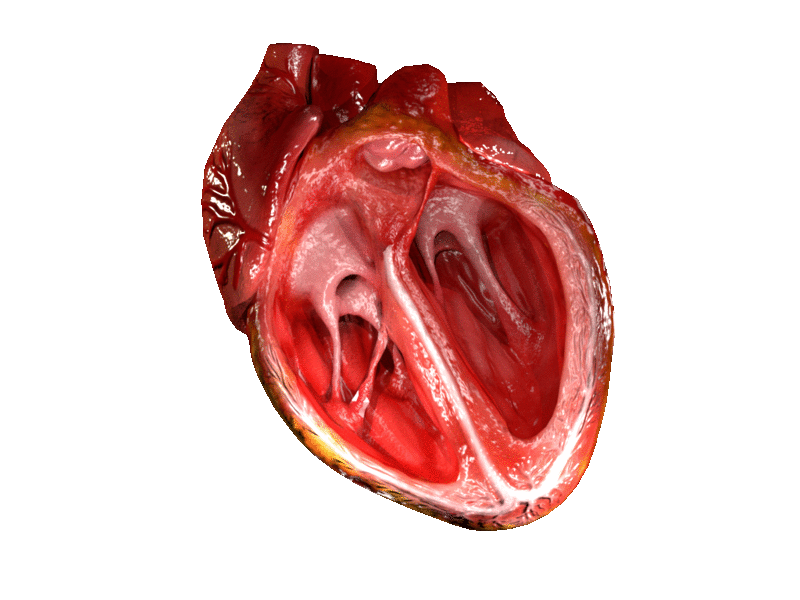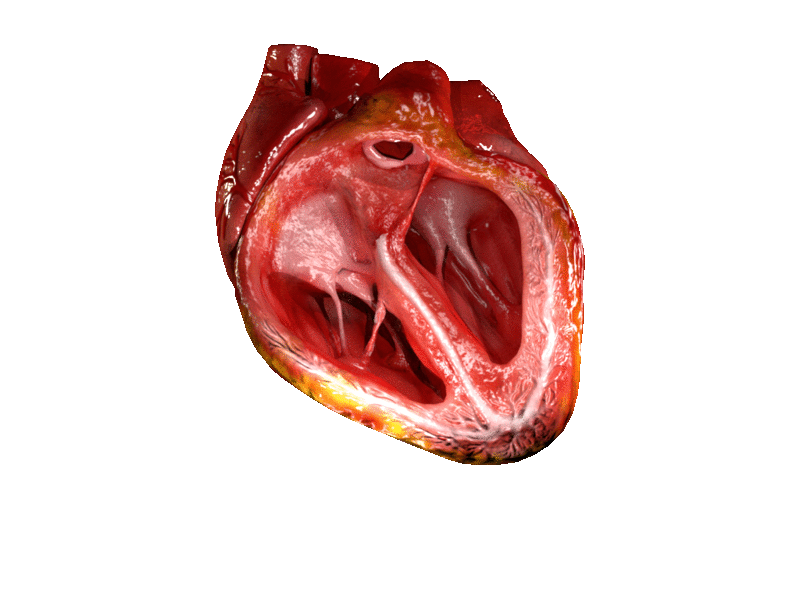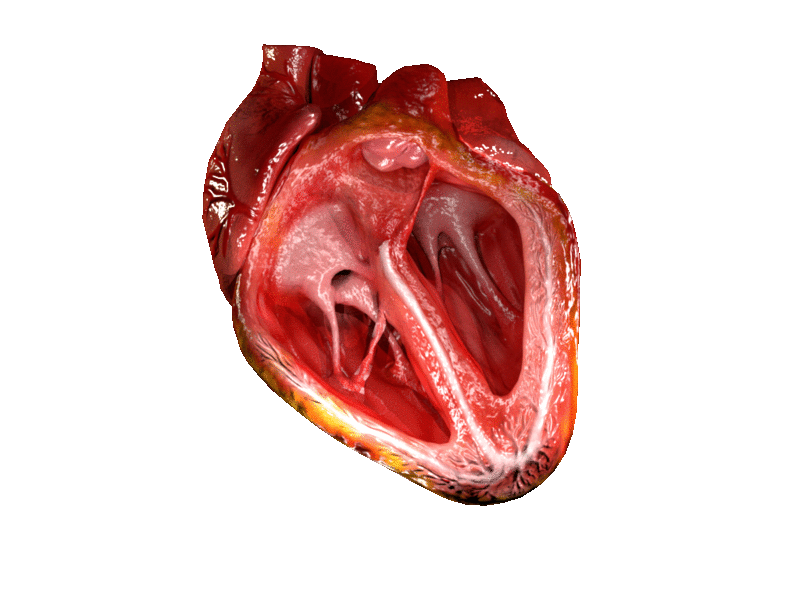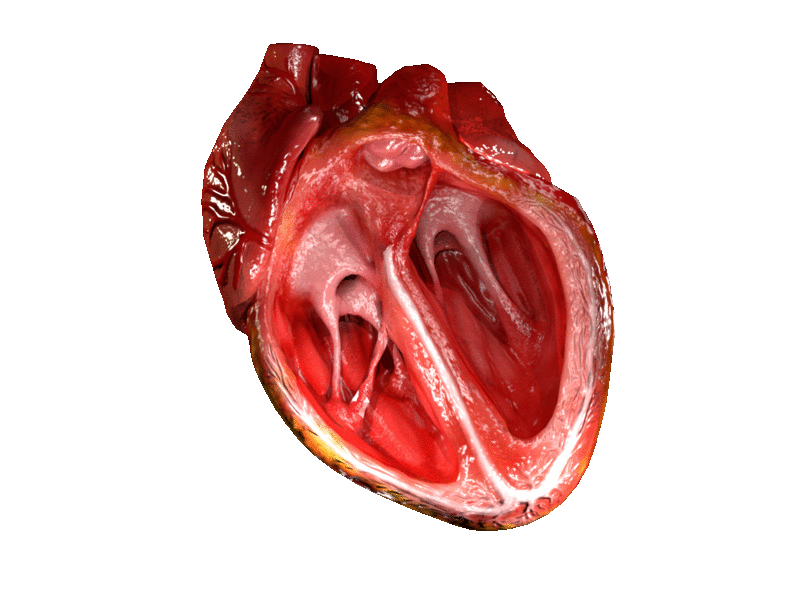
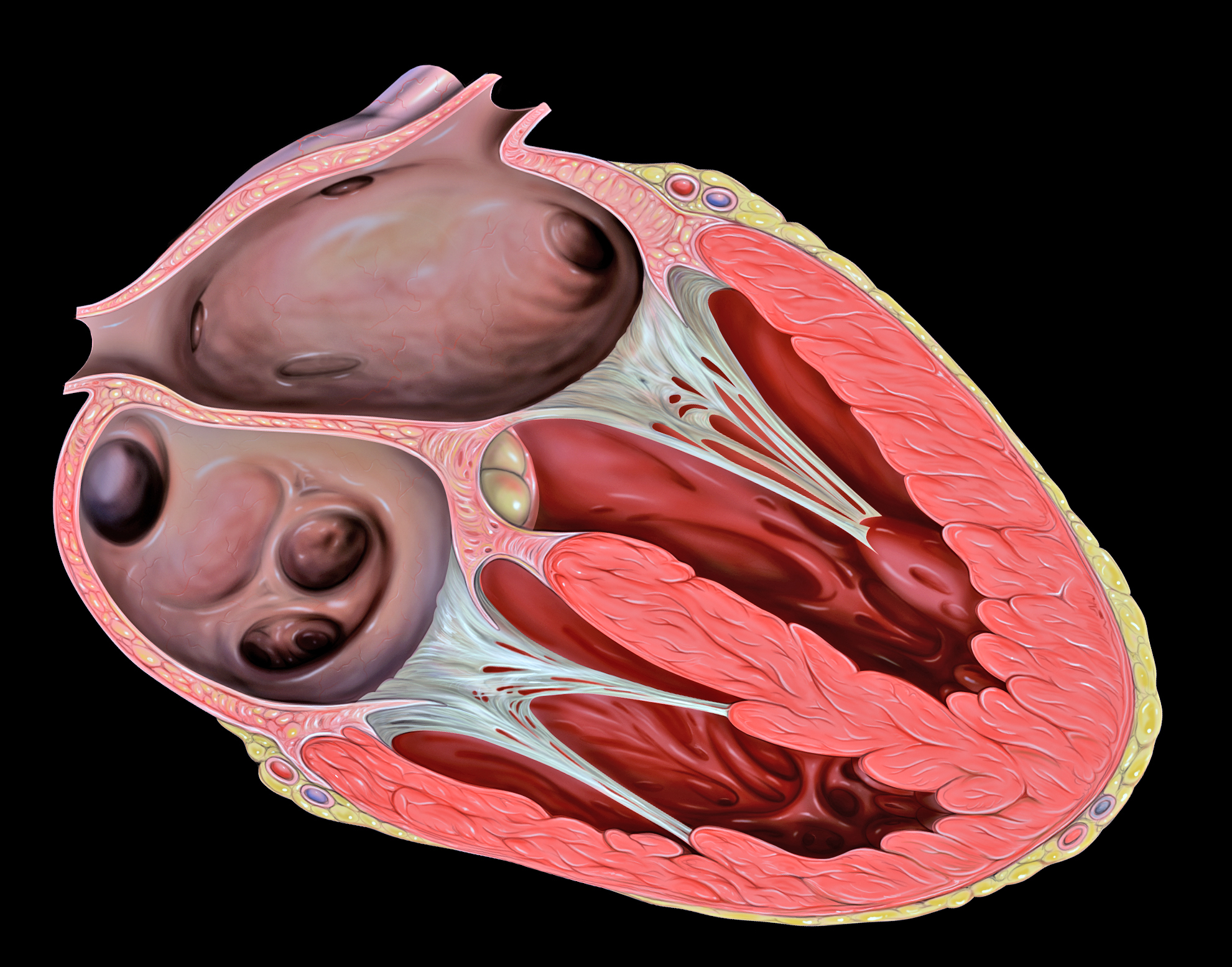
The two atrioventricular (AV) valves prevent backflow when the ventricles are contracting, while the semilunar valves prevent backflow from vessels. This means that the AV valves must withstand much more pressure than do the semilunar valves. In order to withstand the force of the ventricles contracting (to prevent blood from backflowing into the atria), the AV valves are reinforced with structures called chordae tendineae — tendon-like cords of connective tissue which anchor the valve and prevent it from prolapse. Figure 14.3.6 shows the structure and location of the chordae tendoneae.
The chordae tendoneae are under such force that they need special attachments to the interior of the ventricles where they anchor. Papillary muscles are specialized muscles in the interior of the ventricle that provide a strong anchor point for the chordae tendineae.
Coronary Circulation
The cardiomyocytes of the muscular walls of the heart are very active cells, because they are responsible for the constant beating of the heart. These cells need a continuous supply of oxygen and nutrients. The carbon dioxide and waste products they produce also must be continuously removed. The blood vessels that carry blood to and from the heart muscle cells make up the coronary circulation. Note that the blood vessels of the coronary circulation supply heart tissues with blood, and are different from the blood vessels that carry blood to and from the chambers of the heart as part of the general circulation. Coronary arteries supply oxygen-rich blood to the heart muscle cells. Coronary veins remove deoxygenated blood from the heart muscles cells.
- There are two coronary arteries — a right coronary artery that supplies the right side of the heart, and a left coronary artery that supplies the left side of the heart. These arteries branch repeatedly into smaller and smaller arteries and finally into capillaries, which exchange gases, nutrients, and waste products with cardiomyocytes.
- At the back of the heart, small cardiac veins drain into larger veins, and finally into the great cardiac vein, which empties into the right atrium. At the front of the heart, small cardiac veins drain directly into the right atrium.
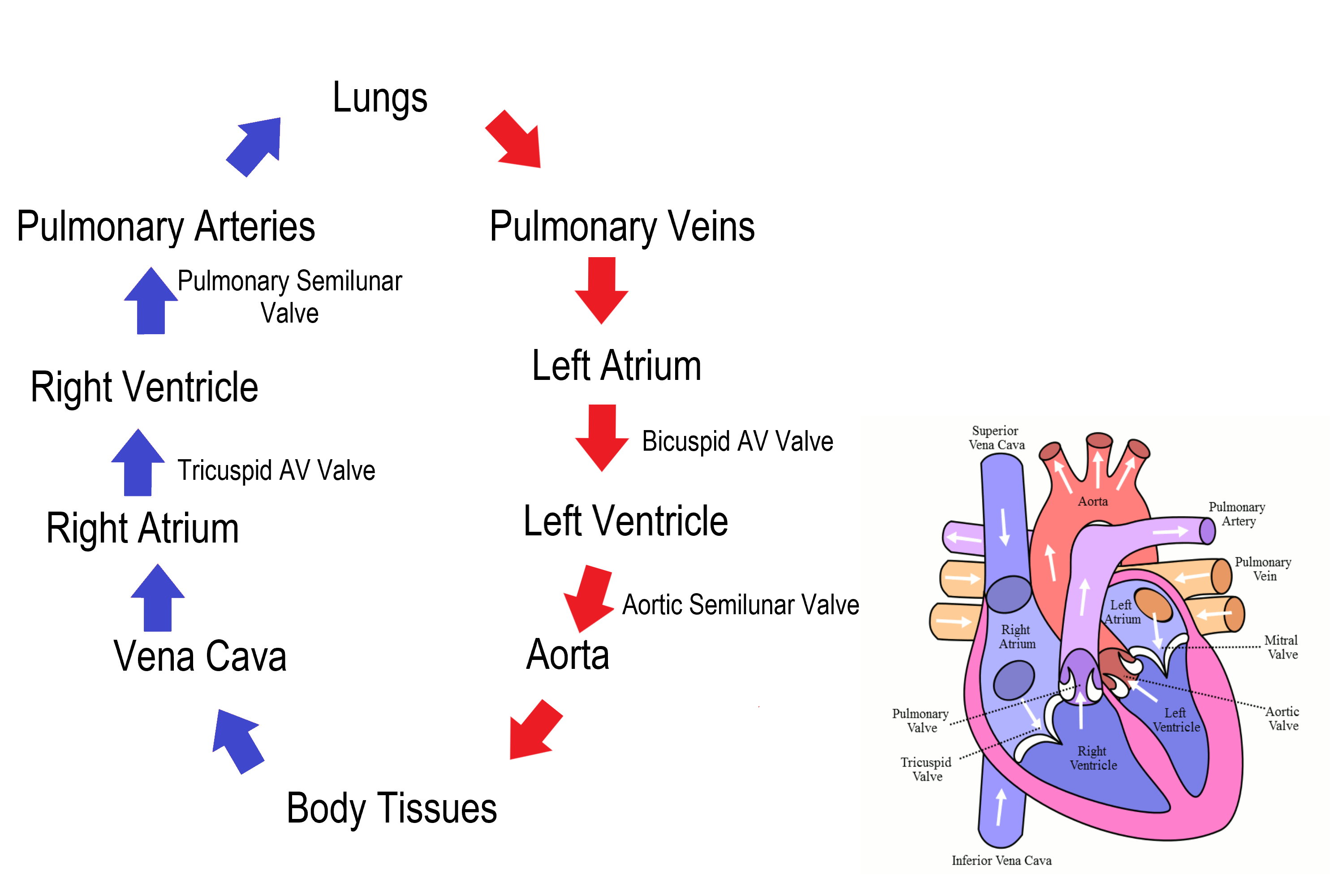
Blood Circulation Through the Heart
Figure 14.3.7 shows how blood circulates through the chambers of the heart. The right atrium collects blood from two large veins, the superior vena cava (from the upper body) and the inferior vena cava (from the lower body). The blood that collects in the right atrium is pumped through the tricuspid valve into the right ventricle. From the right ventricle, the blood is pumped through the pulmonary valve into the pulmonary artery. The pulmonary artery carries the blood to the lungs, where it enters the pulmonary circulation, gives up carbon dioxide, and picks up oxygen. The oxygenated blood travels back from the lungs through the pulmonary veins (of which there are four), and enters the left atrium of the heart. From the left atrium, the blood is pumped through the mitral valve into the left ventricle. From the left ventricle, the blood is pumped through the aortic valve into the aorta, which subsequently branches into smaller arteries that carry the blood throughout the rest of the body. After passing through capillaries and exchanging substances with cells, the blood returns to the right atrium via the superior vena cava and inferior vena cava, and the process begins anew.
Cardiac Cycle
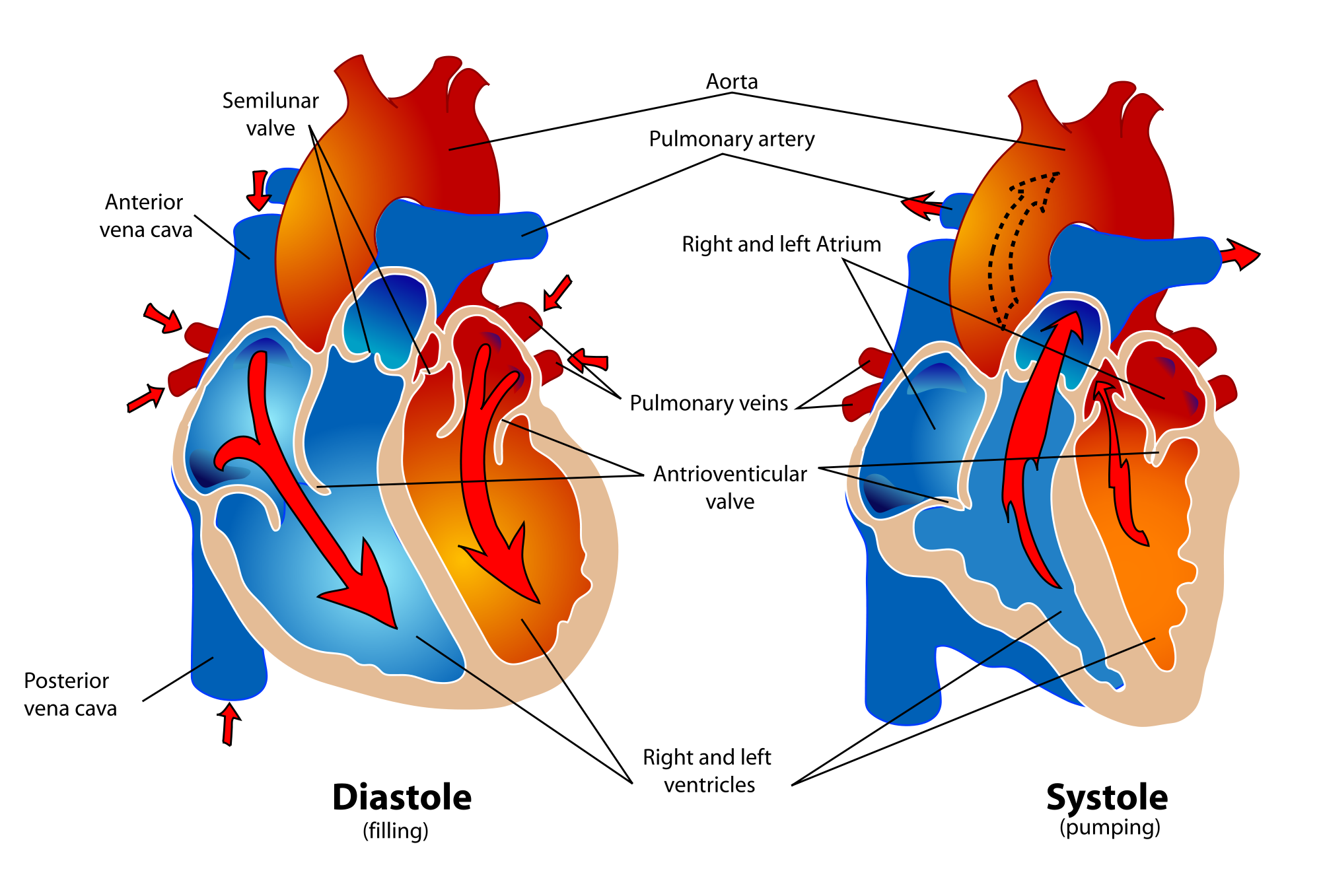
The cardiac cycle refers to a single complete heartbeat, which includes one iteration of the lub and dub sounds heard through a stethoscope. During the cardiac cycle, the atria and ventricles work in a coordinated fashion so that blood is pumped efficiently through and out of the heart. The cardiac cycle includes two parts, called diastole and systole, which are illustrated in the diagrams in Figure 14.3.8.
- During diastole, the atria contract and pump blood into the ventricles, while the ventricles relax and fill with blood from the atria.
- During systole, the atria relax and collect blood from the lungs and body, while the ventricles contract and pump blood out of the heart.
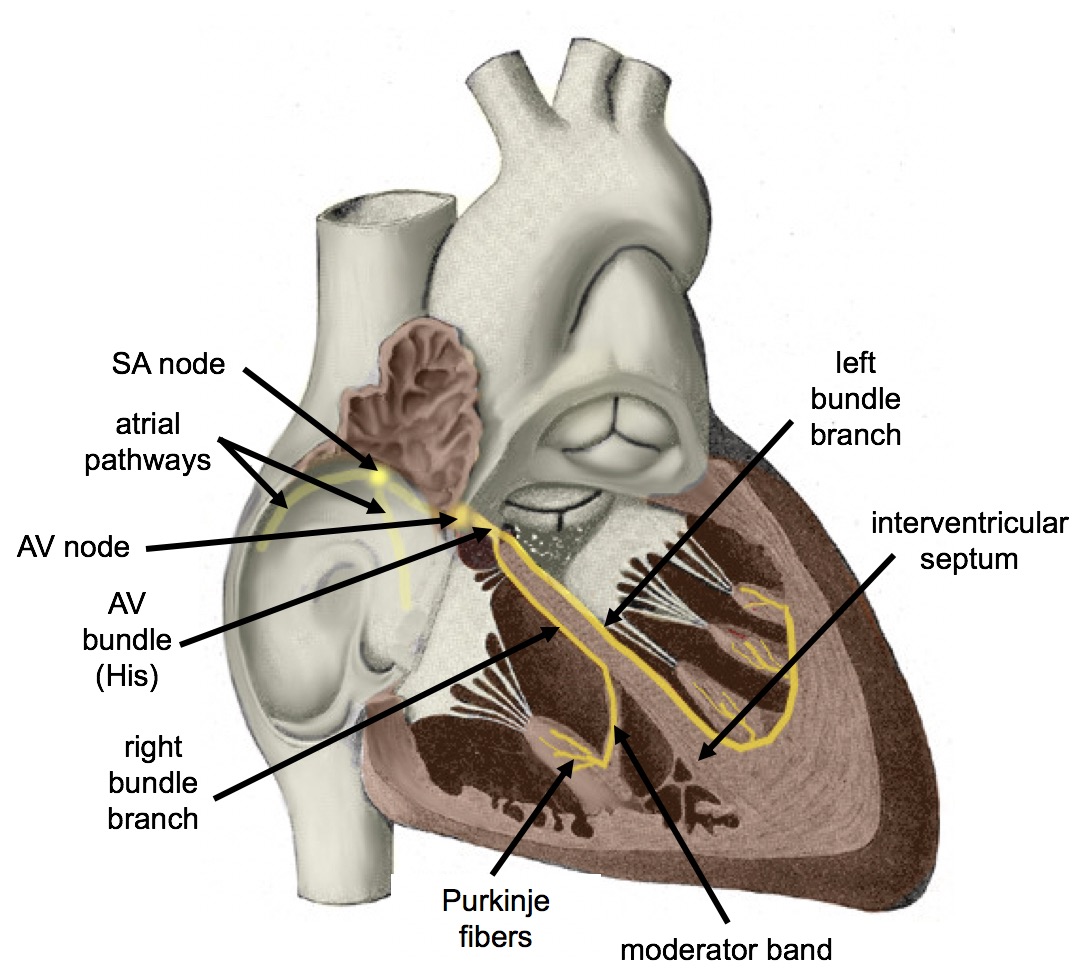
Electrical Stimulation of the Heart
The normal, rhythmical beating of the heart is called sinus rhythm. It is established by the heart’s pacemaker cells, which are located in an area of the heart called the sinoatrial node (shown in Figure 14.3.9). The pacemaker cells create electrical signals with the movement of electrolytes (sodium, potassium, and calcium ions) into and out of the cells. For each cardiac cycle, an electrical signal rapidly travels first from the sinoatrial node, to the right and left atria so they contract together. Then, the signal travels to another node, called the atrioventricular node (Figure 14.3.9), and from there to the right and left ventricles (which also contract together), just a split second after the atria contract.
The normal sinus rhythm of the heart is influenced by the autonomic nervous system through sympathetic and parasympathetic nerves. These nerves arise from two paired cardiovascular centers in the medulla of the brainstem. The parasympathetic nerves act to decrease the heart rate, and the sympathetic nerves act to increase the heart rate. Parasympathetic input normally predominates. Without it, the pacemaker cells of the heart would generate a resting heart rate of about 100 beats per minute, instead of a normal resting heart rate of about 72 beats per minute. The cardiovascular centers receive input from receptors throughout the body, and act through the sympathetic nerves to increase the heart rate, as needed. Increased physical activity, for example, is detected by receptors in muscles, joints, and tendons. These receptors send nerve impulses to the cardiovascular centers, causing sympathetic nerves to increase the heart rate, and allowing more blood to flow to the muscles.
Besides the autonomic nervous system, other factors can also affect the heart rate. For example, thyroid hormones and adrenal hormones (such as epinephrine) can stimulate the heart to beat faster. The heart rate also increases when blood pressure drops or the body is dehydrated or overheated. On the other hand, cooling of the body and relaxation — among other factors — can contribute to a decrease in the heart rate.
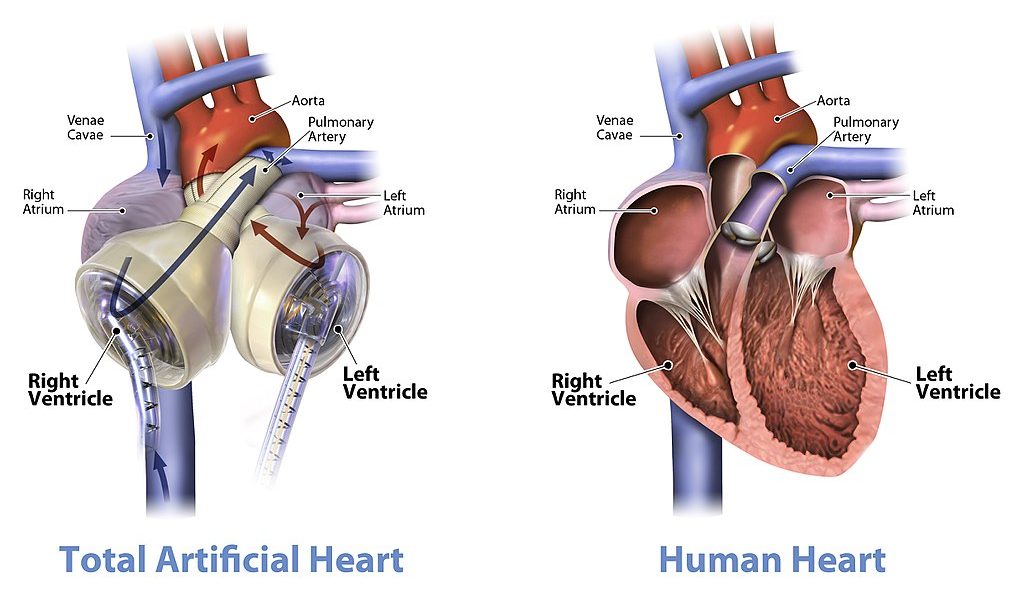
Feature: Human Biology in the News
When a patient’s heart is too diseased or damaged to sustain life, a heart transplant is likely to be the only long-term solution. The first successful heart transplant was undertaken in South Africa in 1967. There are over 2,200 Canadians walking around today because of life-saving heart transplant surgery. Approximately 180 heart transplant surgeries are performed each year, but there are still so many Canadians on the transplant list that some die while waiting for a heart. The problem is that far too few hearts are available for transplant — there is more demand (people waiting for a heart transplant) than supply (organ donors). Sometimes, recipient hopefuls will receive a device called a Total Artificial Heart (see Figure 14.3.10), which can buy them some time until a donor heart becomes available.
Watch the video below "Total artificial heart option..." from Stanford Health Care to see how it works:
https://youtu.be/1PtxaxcPnGc
Total artificial heart option at Stanford (Includes surgical graphic footage), Stanford Health Care, 2014.
14.3 Summary
- The heart is a muscular organ behind the sternum and slightly to the left of the center of the chest. Its function is to pump blood through the blood vessels of the cardiovascular system.
- The wall of the heart consists of three layers. The middle layer, the myocardium, is the thickest layer and consists mainly of cardiac muscle. The interior of the heart consists of four chambers, with an upper atrium and lower ventricle on each side of the heart. Blood enters the heart through the atria, which pump it to the ventricles. Then the ventricles pump blood out of the heart. Four valves in the heart keep blood flowing in the correct direction and prevent backflow.
- The coronary circulation consists of blood vessels that carry blood to and from the heart muscle cells, and is different from the general circulation of blood through the heart chambers. There are two coronary arteries that supply the two sides of the heart with oxygenated blood. Cardiac veins drain deoxygenated blood back into the heart.
- Deoxygenated blood flows into the right atrium through veins from the upper and lower body (superior and inferior vena cava, respectively), and oxygenated blood flows into the left atrium through four pulmonary veins from the lungs. Each atrium pumps the blood to the ventricle below it. From the right ventricle, deoxygenated blood is pumped to the lungs through the two pulmonary arteries. From the left ventricle, oxygenated blood is pumped to the rest of the body through the aorta.
- The cardiac cycle refers to a single complete heartbeat. It includes diastole — when the atria contract — and systole, when the ventricles contract.
- The normal, rhythmic beating of the heart is called sinus rhythm. It is established by the heart’s pacemaker cells in the sinoatrial node. Electrical signals from the pacemaker cells travel to the atria, and cause them to contract. Then, the signals travel to the atrioventricular node and from there to the ventricles, causing them to contract. Electrical stimulation from the autonomic nervous system and hormones from the endocrine system can also influence heartbeat.
14.3 Review Questions
- What is the heart, where is located, and what is its function?
-
- Describe the coronary circulation.
- Summarize how blood flows into, through, and out of the heart.
- Explain what controls the beating of the heart.
- What are the two types of cardiac muscle cells in the myocardium? What are the differences between these two types of cells?
- Explain why the blood from the cardiac veins empties into the right atrium of the heart. Focus on function (rather than anatomy) in your answer.
14.3 Explore More
https://www.youtube.com/watch?v=1bnzVjOJ6NM
Noel Bairey Merz: The single biggest health threat women face, TED, 2012.
https://www.youtube.com/watch?v=jJm7zBcN6-M
Watch a Transcatheter Aortic Valve Replacement (TAVR) Procedure at St. Luke's in Cedar Rapids, Iowa, UnityPoint Health - Cedar Rapids, 2018.
https://www.youtube.com/watch?v=zU6mmix04PI
A Change of Heart: My Transplant Experience | Thomas Volk | TEDxUWLaCrosse, TEDx Talks, 2018.
https://www.youtube.com/watch?v=biGuwQhuAsk
Heart Transplant Recipient Meets Donor Family For The First Time, WMC Health, 2018.
Attributions
Figure 14.3.1
- Female clinician dressed in scrubs using a stethoscope by Amanda Mills, USCDCP, on Pixnio is used under a CC0 public domain certification license (https://creativecommons.org/licenses/publicdomain/).
- Human heart beating loud and strong (audio) by Daniel Simion on Soundbible.com is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 14.3.2
Blausen_0470_HeartWall by BruceBlaus on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 14.3.3
Diagram_of_the_human_heart_(cropped).svg by Wapcaplet on Wikimedia Commons is used under a CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0/) license.
Figure 14.3.4
Heart_Valves by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 14.3.5
CG_Heart Valve Animation by DrJanaOfficial on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
Figure 14.3.6
Heart_tee_four_chamber_view by Patrick J. Lynch, medical illustrator from Yale University School of Medicine, on Wikimedia Commons is used under a CC BY 2.5 (https://creativecommons.org/licenses/by/2.5) license.
Figure 14.3.7
Circulation of blood through the heart by Christinelmiller on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license. [Original image in the bottom right is by Wapcaplet / CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/)]
Figure 14.3.8
Human_healthy_pumping_heart_en.svg by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Common is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 14.3.9
Cardiac_Conduction_System by Cypressvine on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
References
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, June 19). Figure 19.12 Heart valves with the atria and major vessels removed [digital image]. In Anatomy and Physiology (Section 19.1). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/19-1-heart-anatomy#fig-ch20_01_04
Blausen.com Staff. (2014). Medical gallery of Blausen Medical 2014. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436.
Heart and Stroke Foundation of Canada. (n.d.). https://www.heartandstroke.ca/
Sliwa, K., Zilla, P. (2017, December 7). 50th anniversary of the first human heart transplant—How is it seen today? European Heart Journal, 38(46):3402–3404. https://doi.org/10.1093/eurheartj/ehx695
Stanford Health Care. (2014, December 3). Total artificial heart option at Stanford (Includes surgical graphic footage). YouTube. https://www.youtube.com/watch?v=1PtxaxcPnGc&feature=youtu.be
TED. (2012, March 21). Noel Bairey Merz: The single biggest health threat women face. YouTube. https://www.youtube.com/watch?v=1bnzVjOJ6NM&feature=youtu.be
TEDx Talks. (2018, April 18). A change of heart: My transplant experience | Thomas Volk | TEDxUWLaCrosse. YouTube. https://www.youtube.com/watch?v=zU6mmix04PI&feature=youtu.be
UMagazine. (2015, Fall). The cutting edge: Patient first to bridge from experimental total artificial heart to transplant. UCLA Health. https://www.uclahealth.org/u-magazine/patient-first-to-bridge-from-experimental-total-artificial-heart-to-transplant
UnityPoint Health - Cedar Rapids. (2018, February 7). Watch a transcatheter aortic valve replacement (TAVR) Procedure at St. Luke's in Cedar Rapids, Iowa. YouTube. https://www.youtube.com/watch?v=jJm7zBcN6-M&feature=youtu.be
WMC Health. (2018, September 13). Heart transplant recipient meets donor family for the first time. YouTube. https://www.youtube.com/watch?v=biGuwQhuAsk&feature=youtu.be
Created by CK-12 Foundation/Adapted by Christine Miller

Goose Bumps
No doubt you’ve experienced the tiny, hair-raising skin bumps called goose bumps, like those you see in Figure 10.4.1. They happen when you feel chilly. Do you know what causes goose bumps, or why they pop up when you are cold? The answers to these questions involve the layer of skin known as the dermis.
What is the Dermis?
The dermis is the inner of the two major layers that make up the skin, the outer layer being the epidermis. The dermis consists mainly of connective tissues. It also contains most skin structures, such as glands and blood vessels. The dermis is anchored to the tissues below it by flexible collagen bundles that permit most areas of the skin to move freely over subcutaneous (“below the skin”) tissues. Functions of the dermis include cushioning subcutaneous tissues, regulating body temperature, sensing the environment, and excreting wastes.
Anatomy of the Dermis
The basic anatomy of the dermis is a matrix, or sort of scaffolding, composed of connective tissues. These tissues include collagen fibres — which provide toughness — and elastin fibres, which provide elasticity. Surrounding these fibres, the matrix also includes a gel-like substance made of proteins. The tissues of the matrix give the dermis both strength and flexibility.
The dermis is divided into two layers: the papillary layer and the reticular layer. Both layers are shown in Figure 10.4.2 below and described in the text that follows.
Papillary Layer
The papillary layer is the upper layer of the dermis, just below the basement membrane that connects the dermis to the epidermis above it. The papillary layer is the thinner of the two dermal layers. It is composed mainly of loosely arranged collagen fibres. The papillary layer is named for its fingerlike projections — or papillae — that extend upward into the epidermis. The papillae contain capillaries and sensory touch receptors.

The papillae give the dermis a bumpy surface that interlocks with the epidermis above it, strengthening the connection between the two layers of skin. On the palms and soles, the papillae create epidermal ridges. Epidermal ridges on the fingers are commonly called fingerprints (see Figure 10.4.3). Fingerprints are genetically determined, so no two people (other than identical twins) have exactly the same fingerprint pattern. Therefore, fingerprints can be used as a means of identification, for example, at crime scenes. Fingerprints were much more commonly used forensically before DNA analysis was introduced for this purpose.
Reticular Layer
The reticular layer is the lower layer of the dermis, located below the papillary layer. It is the thicker of the two dermal layers. It is composed of densely woven collagen and elastin fibres. These protein fibres give the dermis its properties of strength and elasticity. This layer of the dermis cushions subcutaneous tissues of the body from stress and strain. The reticular layer of the dermis also contains most of the structures in the dermis, such as glands and hair follicles.
Structures in the Dermis
Both papillary and reticular layers of the dermis contain numerous sensory receptors, which make the skin the body’s primary sensory organ for the sense of touch. Both dermal layers also contain blood vessels. They provide nutrients to remove wastes from dermal cells, as well as cells in the lowest layer of the epidermis, the stratum basale. The circulatory components of the dermis are shown in Figure 10.4.4 below.
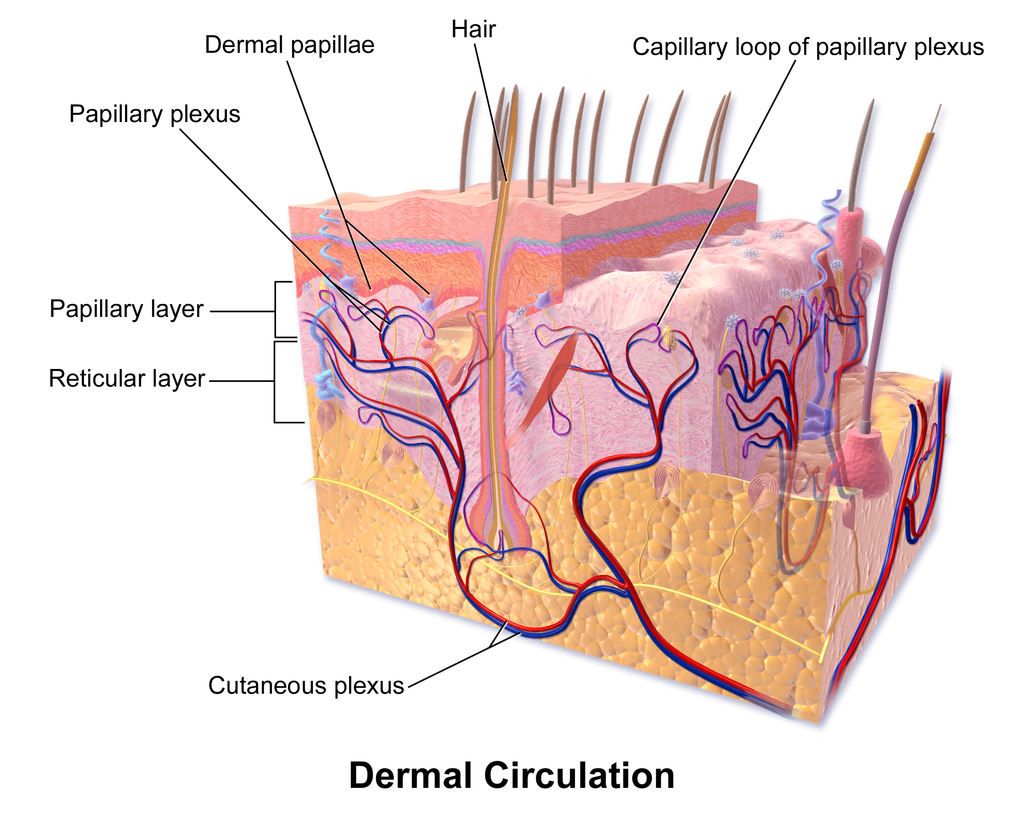
Glands
Glands in the reticular layer of the dermis include sweat glands and sebaceous (oil) glands. Both are exocrine glands, which are glands that release their secretions through ducts to nearby body surfaces. The diagram in Figure 10.4.5 shows these glands, as well as several other structures in the dermis.
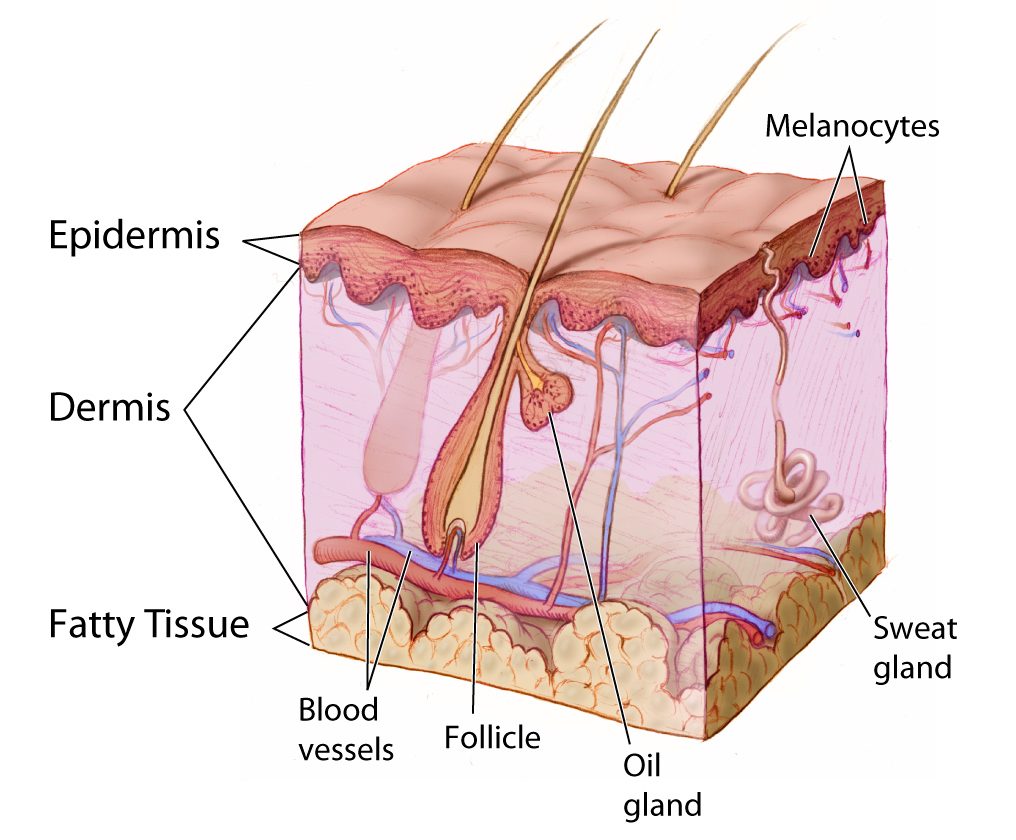
Sweat Glands
Sweat glands produce the fluid called sweat, which contains mainly water and salts. The glands have ducts that carry the sweat to hair follicles, or to the surface of the skin. There are two different types of sweat glands: eccrine glands and apocrine glands.
- Eccrine sweat glands occur in skin all over the body. Their ducts empty through tiny openings called pores onto the skin surface. These sweat glands are involved in temperature regulation.
- Apocrine sweat glands are larger than eccrine glands, and occur only in the skin of the armpits and groin. The ducts of apocrine glands empty into hair follicles, and then the sweat travels along hairs to reach the surface. Apocrine glands are inactive until puberty, at which point they start producing an oily sweat that is consumed by bacteria living on the skin. The digestion of apocrine sweat by bacteria causes body odor.
Sebaceous Glands
Sebaceous glands are exocrine glands that produce a thick, fatty substance called sebum. Sebum is secreted into hair follicles and makes its way to the skin surface along hairs. It waterproofs the hair and skin, and helps prevent them from drying out. Sebum also has antibacterial properties, so it inhibits the growth of microorganisms on the skin. Sebaceous glands are found in every part of the skin — except for the palms of the hands and soles of the feet, where hair does not grow.
Hair Follicles
Hair follicles are the structures where hairs originate (see the diagram above). Hairs grow out of follicles, pass through the epidermis, and exit at the surface of the skin. Associated with each hair follicle is a sebaceous gland, which secretes sebum that coats and waterproofs the hair. Each follicle also has a bed of capillaries, a nerve ending, and a tiny muscle called an arrector pili.
Functions of the Dermis
The main functions of the dermis are regulating body temperature, enabling the sense of touch, and eliminating wastes from the body.
Temperature Regulation
Several structures in the reticular layer of the dermis are involved in regulating body temperature. For example, when body temperature rises, the hypothalamus of the brain sends nerve signals to sweat glands, causing them to release sweat. An adult can sweat up to four litres an hour. As the sweat evaporates from the surface of the body, it uses energy in the form of body heat, thus cooling the body. The hypothalamus also causes dilation of blood vessels in the dermis when body temperature rises. This allows more blood to flow through the skin, bringing body heat to the surface, where it can radiate into the environment.
When the body is too cool, sweat glands stop producing sweat, and blood vessels in the skin constrict, thus conserving body heat. The arrector pili muscles also contract, moving hair follicles and lifting hair shafts. This results in more air being trapped under the hairs to insulate the surface of the skin. These contractions of arrector pili muscles are the cause of goose bumps.
Sensing the Environment
Sensory receptors in the dermis are mainly responsible for the body’s tactile senses. The receptors detect such tactile stimuli as warm or cold temperature, shape, texture, pressure, vibration, and pain. They send nerve impulses to the brain, which interprets and responds to the sensory information. Sensory receptors in the dermis can be classified on the basis of the type of touch stimulus they sense. Mechanoreceptors sense mechanical forces such as pressure, roughness, vibration, and stretching. Thermoreceptors sense variations in temperature that are above or below body temperature. Nociceptors sense painful stimuli. Figure 10.4.6 shows several specific kinds of tactile receptors in the dermis. Each kind of receptor senses one or more types of touch stimuli.
- Free nerve endings sense pain and temperature variations.
- Merkel cells sense light touch, shapes, and textures.
- Meissner’s corpuscles sense light touch.
- Pacinian corpuscles sense pressure and vibration.
- Ruffini corpuscles sense stretching and sustained pressure.
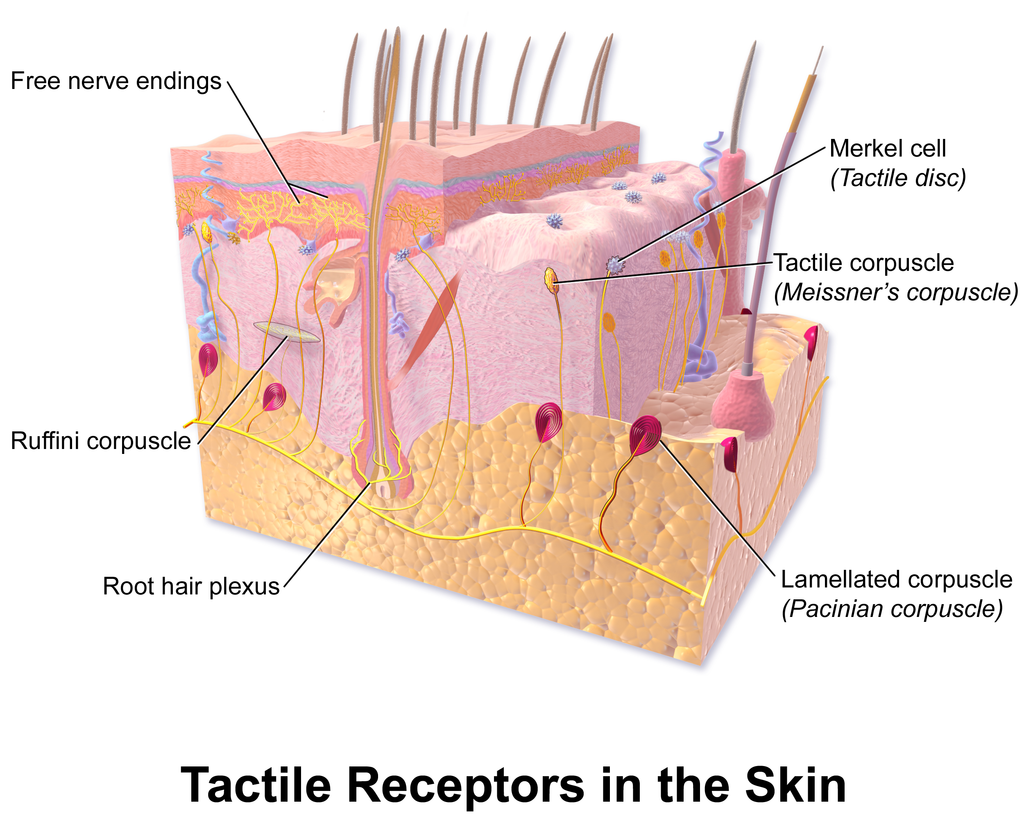
Excreting Wastes
The sweat released by eccrine sweat glands is one way the body excretes waste products. Sweat contains excess water, salts (electrolytes), and other waste products that the body must get rid of to maintain homeostasis. The most common electrolytes in sweat are sodium and chloride. Potassium, calcium, and magnesium electrolytes may be excreted in sweat, as well. When these electrolytes reach high levels in the blood, more are excreted in sweat. This helps to bring their blood levels back into balance. Besides electrolytes, sweat contains small amounts of waste products from metabolism, including ammonia and urea. Sweat may also contain alcohol in someone who has been drinking alcoholic beverages.
Feature: My Human Body
Acne is the most common skin disorder in the Canada. At least 20% of Canadians have acne at any given time and it affects approximately 90% of adolescents (as in Figure 10.4.7). Although acne occurs most commonly in teens and young adults, but it can occur at any age. Even newborn babies can get acne.
The main sign of acne is the appearance of pimples (pustules) on the skin, like those in the photo above. Other signs of acne may include whiteheads, blackheads, nodules, and other lesions. Besides the face, acne can appear on the back, chest, neck, shoulders, upper arms, and buttocks. Acne can permanently scar the skin, especially if it isn’t treated appropriately. Besides its physical effects on the skin, acne can also lead to low self-esteem and depression.
Acne is caused by clogged, sebum-filled pores that provide a perfect environment for the growth of bacteria. The bacteria cause infection, and the immune system responds with inflammation. Inflammation, in turn, causes swelling and redness, and may be associated with the formation of pus. If the inflammation goes deep into the skin, it may form an acne nodule.
Mild acne often responds well to treatment with over-the-counter (OTC) products containing benzoyl peroxide or salicylic acid. Treatment with these products may take a month or two to clear up the acne. Once the skin clears, treatment generally needs to continue for some time to prevent future breakouts.
If acne fails to respond to OTC products, nodules develop, or acne is affecting self-esteem, a visit to a dermatologist is in order. A dermatologist can determine which treatment is best for a given patient. A dermatologist can also prescribe prescription medications (which are likely to be more effective than OTC products) and provide other medical treatments, such as laser light therapies or chemical peels.
What can you do to maintain healthy skin and prevent or reduce acne? Dermatologists recommend the following tips:
- Wash affected or acne-prone skin (such as the face) twice a day, and after sweating.
- Use your fingertips to apply a gentle, non-abrasive cleanser. Avoid scrubbing, which can make acne worse.
- Use only alcohol-free products and avoid any products that irritate the skin, such as harsh astringents or exfoliants.
- Rinse with lukewarm water, and avoid using very hot or cold water.
- Shampoo your hair regularly.
- Do not pick, pop, or squeeze acne. If you do, it will take longer to heal and is more likely to scar.
- Keep your hands off your face. Avoid touching your skin throughout the day.
- Stay out of the sun and tanning beds. Some acne medications make your skin very sensitive to UV light.
10.4 Summary
- The dermis is the inner and thicker of the two major layers that make up the skin. It consists mainly of a matrix of connective tissues that provide strength and stretch. It also contains almost all skin structures, including sensory receptors and blood vessels.
- The dermis has two layers. The upper papillary layer has papillae extending upward into the epidermis and loose connective tissues. The lower reticular layer has denser connective tissues and structures, such as glands and hair follicles. Glands in the dermis include eccrine and apocrine sweat glands and sebaceous glands. Hair follicles are structures where hairs originate.
- Functions of the dermis include cushioning subcutaneous tissues, regulating body temperature, sensing the environment, and excreting wastes. The dense connective tissues of the dermis provide cushioning. The dermis regulates body temperature mainly by sweating and by vasodilation or vasoconstriction. The many tactile sensory receptors in the dermis make it the main organ for the sense of touch. Wastes excreted in sweat include excess water, electrolytes, and certain metabolic wastes.
10.4 Review Questions
- What is the dermis?
- Describe the basic anatomy of the dermis.
- Compare and contrast the papillary and reticular layers of the dermis.
- What causes epidermal ridges, and why can they be used to identify individuals?
- Name the two types of sweat glands in the dermis, and explain how they differ.
- What is the function of sebaceous glands?
- Describe the structures associated with hair follicles.
- Explain how the dermis helps regulate body temperature.
- Identify three specific kinds of tactile receptors in the dermis, along with the type of stimuli they sense.
- How does the dermis excrete wastes? What waste products does it excrete?
- What are subcutaneous tissues? Which layer of the dermis provides cushioning for subcutaneous tissues? Why does this layer provide most of the cushioning, instead of the other layer?
- For each of the functions listed below, describe which structure within the dermis carries it out.
- Brings nutrients to and removes wastes from dermal and lower epidermal cells
- Causes hairs to move
- Detects painful stimuli on the skin
10.4 Explore More
https://www.youtube.com/watch?v=FX-FwK0IIrE
How do you get rid of acne? SciShow, 2016.
https://www.youtube.com/watch?v=VcHQWMAClhQ&feature=emb_logo
When You Can't Scratch Away An Itch, Seeker, 2013.
Attributions
Figure 10.4.1
Goose_bumps by EverJean on Wikimedia Commons is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0) license.
Figure 10.4.2
Layers_of_the_Dermis by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 10.4.3
Fingerprint_detail_on_male_finger_in_Třebíč,_Třebíč_District by Frettie on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 10.4.4
Blausen_0802_Skin_Dermal Circulation by BruceBlaus on Wikimedia commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 10.4.5
Anatomy_The_Skin_-_NCI_Visuals_Online by Don Bliss (artist) / National Cancer Institute (National Institutes of Health, with the ID 4604) is in the public domain (https://en.wikipedia.org/wiki/public_domain).
Figure 10.4.6
Blausen_0809_Skin_TactileReceptors by BruceBlaus on Wikimedia commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 10.4.7
Akne-jugend by Ellywa on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/public_domain). (No machine-readable author provided. Ellywa assumed, based on copyright claims).
References
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, June 19). Figure 5.7 Layers of the dermis [digital image]. In Anatomy and Physiology (Section 5.1 Layers of the skin). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/5-1-layers-of-the-skin
Blausen.com staff. (2014). Medical gallery of Blausen Medical 2014. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436.
SciShow. (2016, October 26). How do you get rid of acne? YouTube. https://www.youtube.com/watch?v=FX-FwK0IIrE
Seeker. (2013, October 26). When you can't scratch away an itch. YouTube. https://www.youtube.com/watch?v=VcHQWMAClhQ&feature=emb_logo
Created by CK-12/Adapted by Christine Miller
So Many Species!

The collage shows a single species in each of the six kingdoms into which all of Earth's living things are commonly classified. How many species are there in each kingdom? In a word: millions. A total of almost two million living species have already been identified, and new species are being discovered all the time. Scientists estimate that there may be as many as 30 million unique species alive on Earth today! Clearly, there is a tremendous variety of life on Earth.
What Is Biodiversity?
Biological diversity, or biodiversity, refers to all of the variety of life that exists on Earth. Biodiversity can be described and measured at three different levels: species diversity, genetic diversity, and ecosystem diversity.
- Species diversity refers to the number of different species in an ecosystem or on Earth as a whole. This is the most common way to measure biodiversity. Current estimates for Earth's total number of living species range from 5 to 30 million species.
- Genetic diversity refers to the variation in genes within all of these species.
- Ecosystem diversity refers to the variety of ecosystems on Earth. An ecosystem is a system formed by populations of many different species interacting with each other and their environment.
https://www.youtube.com/watch?v=GK_vRtHJZu4
Why is Biodiversity So Important? - Kim Preshoff, TEDEd, 2015
Defining a Species
Biodiversity is most often measured by counting species, but what is a species? The answer to that question is not as straightforward as you might think. Formally, a species is defined as a group of actually or potentially interbreeding organisms. This means that members of the same species are similar enough to each other to produce fertile offspring together. By this definition of species, all human beings alive today belong to one species, Homo sapiens. All humans can potentially interbreed with each other, but not with members of any other species.
In the real world, it isn't always possible to make the observations necessary to determine whether or not different organisms can interbreed. For one thing, many species reproduce asexually, so individuals never interbreed — even with members of their own species. When studying extinct species represented by fossils, it is usually impossible to know if different organisms could interbreed. Keep in mind that 99 per cent of all species that have ever existed are now extinct! In practice, many biologists and virtually all paleontologists generally define species on the basis of morphology, rather than breeding behavior. Morphology refers to the form and structure of organisms. For classification purposes, it generally refers to relatively obvious physical traits. Typically, the more similar to one another different organisms appear, the greater the chance that they will be classified in the same species.
Classifying Living Things
People have been trying to classify the tremendous diversity of life on Earth for more than two thousand years. The science of classifying organisms is called taxonomy. Classification is an important step in understanding the present diversity and past evolutionary history of life on Earth. It helps us make sense of the overwhelming diversity of living things.
Linnaean Classification
All modern classification systems have their roots in the Linnaean classification system, which was developed by Swedish botanist Carolus Linnaeus in the 1700s. He tried to classify all living things known in his time by grouping together organisms that s
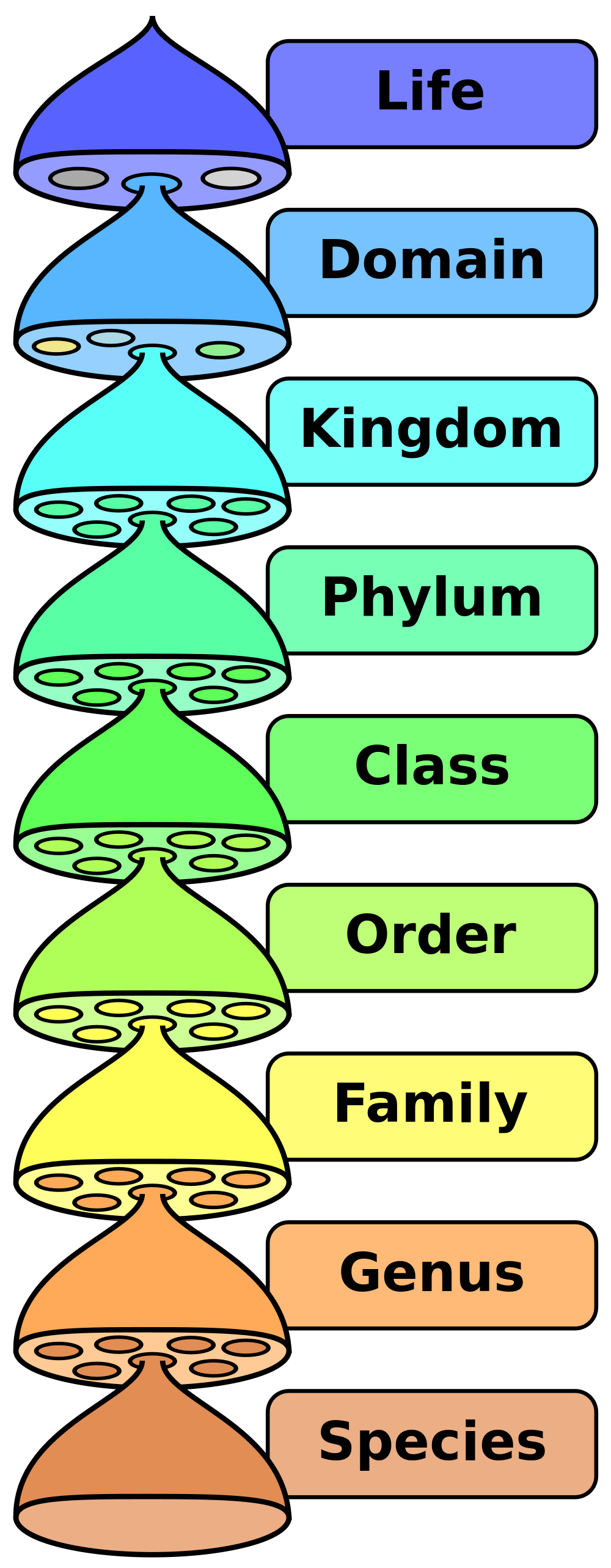
hared obvious morphological traits, such as number of legs or shape of leaves. For his contribution, Linnaeus is known as the “father of taxonomy.”
The Linnaean system of classification consists of a hierarchy of groupings, called taxa (singular, taxon). In the original system, taxa ranged from the kingdom to the species. The kingdom (ex. plant kingdom, animal kingdom) is the largest and most inclusive grouping. It consists of organisms that share just a few basic similarities. The species is the smallest and most exclusive grouping. Ideally, it consists of organisms that are similar enough to interbreed, as discussed above. Similar species are classified together in the same genus (plural, genera), then similar genera are classified together in the same family, and so on, all the way up to the kingdom.
A phrase to help you remember the order of the groupings is shown below. The first letter of each word is the first letter of the level of classification.
Dad Keeps Pots Clean Or Family Gets Sick
The hierarchy of taxa in the original Linnaean system of taxonomy included taxa from the species to the kingdom. The domain was added later.
Binomial Nomenclature
Perhaps the single greatest contribution Linnaeus made to science was his method of naming species. This method, called binomial nomenclature, gives each species a unique, two-word Latin name consisting of the genus name followed by a specific species identifier. An example is Homo sapiens, the two-word Latin name for humans. It literally means “wise human.” This is a reference to our big brains.
Why is having two names so important? It is similar to people having a first and a last name. You may know several people with the first name Michael, but adding Michael’s last name usually pins down exactly which Michael you mean. In the same way, having two names for a species helps to uniquely identify it.
Revisions in the Linnaean Classification
Linnaeus published his classification system in the 1700s. Since then, many new species have been discovered. Scientists can also now classify organisms on the basis of their biochemical and genetic similarities and differences, and not just their outward morphology. These changes have led to revisions in the original Linnaean system of classification.
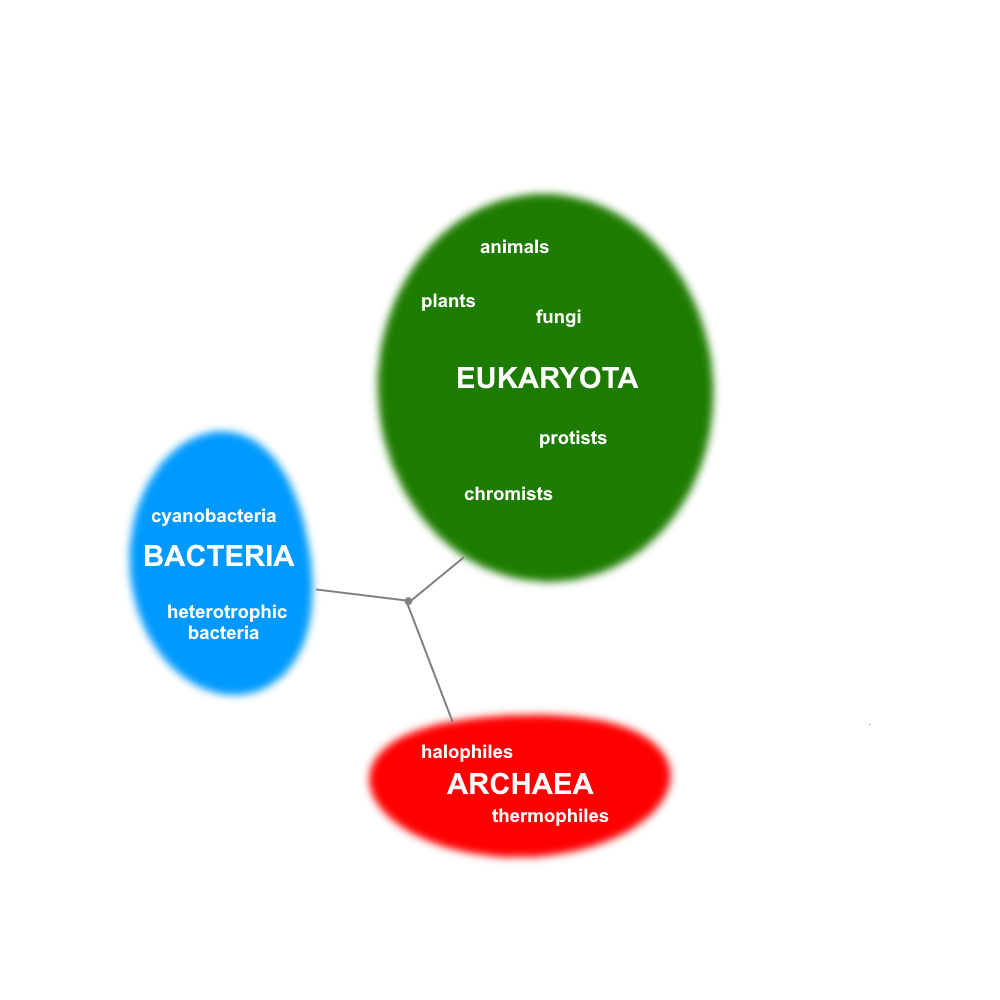
A major change to the Linnaean system is the addition of a new taxon called the domain. The domain is a taxon that is larger and more inclusive than the kingdom, as shown in the figure above. Most biologists agree that there are three domains of life on Earth: Bacteria, Archaea, and Eukarya . Both the Bacteria and the Archaea domains consist of single-celled organisms that lack a nucleus. This means that their genetic material is not enclosed within a membrane inside the cell. The Eukarya domain, in contrast, consists of all organisms whose cells do have a nucleus, so that their genetic material is enclosed within a membrane inside the cell. The Eukarya domain is made up of both single-celled and multicellular organisms. This domain includes several kingdoms, including the animal, plant, fungus, and protist kingdoms.
The three domains of life, as well as how they are related to each other and to a common ancestor. There are several theories about how the three domains are related and which arose first, or from another.
Phylogenetic Classification
Linnaeus classified organisms based on morphology. Basically, organisms were grouped together if they looked alike. After Darwin published his theory of evolution in the 1800s, scientists looked for a way to classify organisms that accounted for phylogeny. Phylogeny is the evolutionary history of a group of related organisms. It is represented by a phylogenetic tree, or some other tree-like diagram, like the one shown above to illustrate the three domains. A phylogenetic tree shows how closely related different groups of organisms are to one another. Each branching point represents a common ancestor of the branching groups.
2.4 Summary
- Biodiversity refers to the variety of life that exists on Earth. It includes species diversity, genetic diversity (within species), and ecosystem diversity.
- The formal biological definition of species is a group of actually or potentially interbreeding organisms. Our own species, Homo sapiens,is an example. In reality, organisms are often classified into species on the basis of morphology.
- A system for classifying living things was introduced by Linnaeus in the 1700s. It includes taxa from the species (least inclusive) to the kingdom (most inclusive). Linnaeus also introduced a system of naming species, which is called binomial nomenclature.
- The domain — a taxon higher than the kingdom — was later added to the Linnaean system. Living things are generally grouped into three domains: Bacteria, Archaea, and Eukarya. The human species and other animal species are placed in the Eukarya domain.
- Modern systems of classification take into account phylogenies, or evolutionary histories of related organisms, rather than just morphological similarities and differences. These relationships are often represented by phylogenetic trees or other tree-like diagrams
2.4 Review Questions
- What is biodiversity? Identify three ways that biodiversity may be measured.
- Define biological species. Why is this definition often difficult to apply?
- Explain why it is important to classify living things, and outline the Linnaean system of classification.
- What is binomial nomenclature? Give an example.
-
- Contrast the Linnaean and phylogenetic systems of classification.
- Describe the taxon called the domain, and compare the three widely recognized domains of living things.
- Based on the phylogenetic tree for the three domains of life above, explain whether you think Bacteria are more closely related to Archaea or Eukarya.
- A scientist discovers a new single-celled organism. Answer the following questions about this discovery.
- If this is all you know, can you place the organism into a particular domain? If so, what is the domain? If not, why not?
- What is one type of information that could help the scientist classify the organism?
- Define morphology. Give an example of a morphological trait in humans.
- Which type of biodiversity is represented in the differences between humans?
- Why do you think it is important to the definition of a species that members of a species can produce fertile offspring?
- Go to the A-Z Animals Animal Classification Page. In the search box, put in your favorite animal and write out it's classification.
2.4 Explore More
https://youtu.be/DVouQRAKxYo
Classification, Amoeba Sisters, 2013.
Attributions
Figure 2.4.1 (6 Kingdoms collage)
- Salmonella, by unknown/ NIAID on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
- Fern from pxhere, is used under a CC0 1.0 universal public domain dedication license (https://creativecommons.org/publicdomain/zero/1.0/deed.en).
- Photo [squirrel] , by Radoslaw Prekurat on Unsplash is used under the Unsplash License (https://unsplash.com/license).
- Blood Milk Mushroom by Hans on Pixabay is used under the Pixabay License (https://pixabay.com/de/service/license/).
- Fungi by Ste Wright on Unsplash is used under the Unsplash License (https://unsplash.com/license).
- EscherichiaColi NIAID [adapted], by Rocky Mountain Laboratories, ca:NIAID, ca:NIH on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 2.4.2
Biological classification, by Pengo [Peter Halasz] on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 2.4.3
The three domains of life and major groups within, by C. Miller, 2019, is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
References
Amoeba Sisters. (2017, March 8). Classification. YouTube. https://www.youtube.com/watch?v=DVouQRAKxYo&feature=youtu.be
A-Z Animals. (2008, December 1). Animal classification. https://a-z-animals.com/reference/animal-classification/
TED-Ed. (2015, April 20). Why is biodiversity so important? - Kim Preshoff. YouTube. https://www.youtube.com/watch?v=GK_vRtHJZu4
Wikipedia contributors. (2020, June 21). Carl Linnaeus. Wikipedia. https://en.wikipedia.org/w/index.php?title=Carl_Linnaeus&oldid=963767022
Created by CK-12 Foundation/Adapted by Christine Miller

Doing the ‘Fly
The swimmer in the Figure 13.3.1 photo is doing the butterfly stroke, a swimming style that requires the swimmer to carefully control his breathing so it is coordinated with his swimming movements. Breathing is the process of moving air into and out of the lungs, which are the organs in which gas exchange takes place between the atmosphere and the body. Breathing is also called ventilation, and it is one of two parts of the life-sustaining process of respiration. The other part is gas exchange. Before you can understand how breathing is controlled, you need to know how breathing occurs.
How Breathing Occurs
Breathing is a two-step process that includes drawing air into the lungs, or inhaling, and letting air out of the lungs, or exhaling. Both processes are illustrated in Figure 13.3.2.
Inhaling
Inhaling is an active process that results mainly from contraction of a muscle called the diaphragm, shown in Figure 13.3.2. The diaphragm is a large, dome-shaped muscle below the lungs that separates the thoracic (chest) and abdominal cavities. When the diaphragm contracts it moves down causing the thoracic cavity to expand, and the contents of the abdomen to be pushed downward. Other muscles — such as intercostal muscles between the ribs — also contribute to the process of inhalation, especially when inhalation is forced, as when taking a deep breath. These muscles help increase thoracic volume by expanding the ribs outward. The increase in thoracic volume creates a decrease in thoracic air pressure. With the chest expanded, there is lower air pressure inside the lungs than outside the body, so outside air flows into the lungs via the respiratory tract according the the pressure gradient (high pressure flows to lower pressure).
Exhaling
Exhaling involves the opposite series of events. The diaphragm relaxes, so it moves upward and decreases the volume of the thorax. Air pressure inside the lungs increases, so it is higher than the air pressure outside the lungs. Exhalation, unlike inhalation, is typically a passive process that occurs mainly due to the elasticity of the lungs. With the change in air pressure, the lungs contract to their pre-inflated size, forcing out the air they contain in the process. Air flows out of the lungs, similar to the way air rushes out of a balloon when it is released. If exhalation is forced, internal intercostal and abdominal muscles may help move the air out of the lungs.
Control of Breathing
Breathing is one of the few vital bodily functions that can be controlled consciously, as well as unconsciously. Think about using your breath to blow up a balloon. You take a long, deep breath, and then you exhale the air as forcibly as you can into the balloon. Both the inhalation and exhalation are consciously controlled.
Conscious Control of Breathing
You can control your breathing by holding your breath, slowing your breathing, or hyperventilating, which is breathing more quickly and shallowly than necessary. You can also exhale or inhale more forcefully or deeply than usual. Conscious control of breathing is common in many activities besides blowing up balloons, including swimming, speech training, singing, playing many different musical instruments (Figure 13.3.3), and doing yoga, to name just a few.
There are limits on the conscious control of breathing. For example, it is not possible for a healthy person to voluntarily stop breathing indefinitely. Before long, there is an irrepressible urge to breathe. If you were able to stop breathing for a long enough time, you would lose consciousness. The same thing would happen if you were to hyperventilate for too long. Once you lose consciousness so you can no longer exert conscious control over your breathing, involuntary control of breathing takes over.
Unconscious Control of Breathing
Unconscious breathing is controlled by respiratory centers in the medulla and pons of the brainstem (see Figure 13.3.4). The respiratory centers automatically and continuously regulate the rate of breathing based on the body’s needs. These are determined mainly by blood acidity, or pH. When you exercise, for example, carbon dioxide levels increase in the blood, because of increased cellular respiration by muscle cells. The carbon dioxide reacts with water in the blood to produce carbonic acid, making the blood more acidic, so pH falls. The drop in pH is detected by chemoreceptors in the medulla. Blood levels of oxygen and carbon dioxide, in addition to pH, are also detected by chemoreceptors in major arteries, which send the “data” to the respiratory centers. The latter respond by sending nerve impulses to the diaphragm, “telling” it to contract more quickly so the rate of breathing speeds up. With faster breathing, more carbon dioxide is released into the air from the blood, and blood pH returns to the normal range.
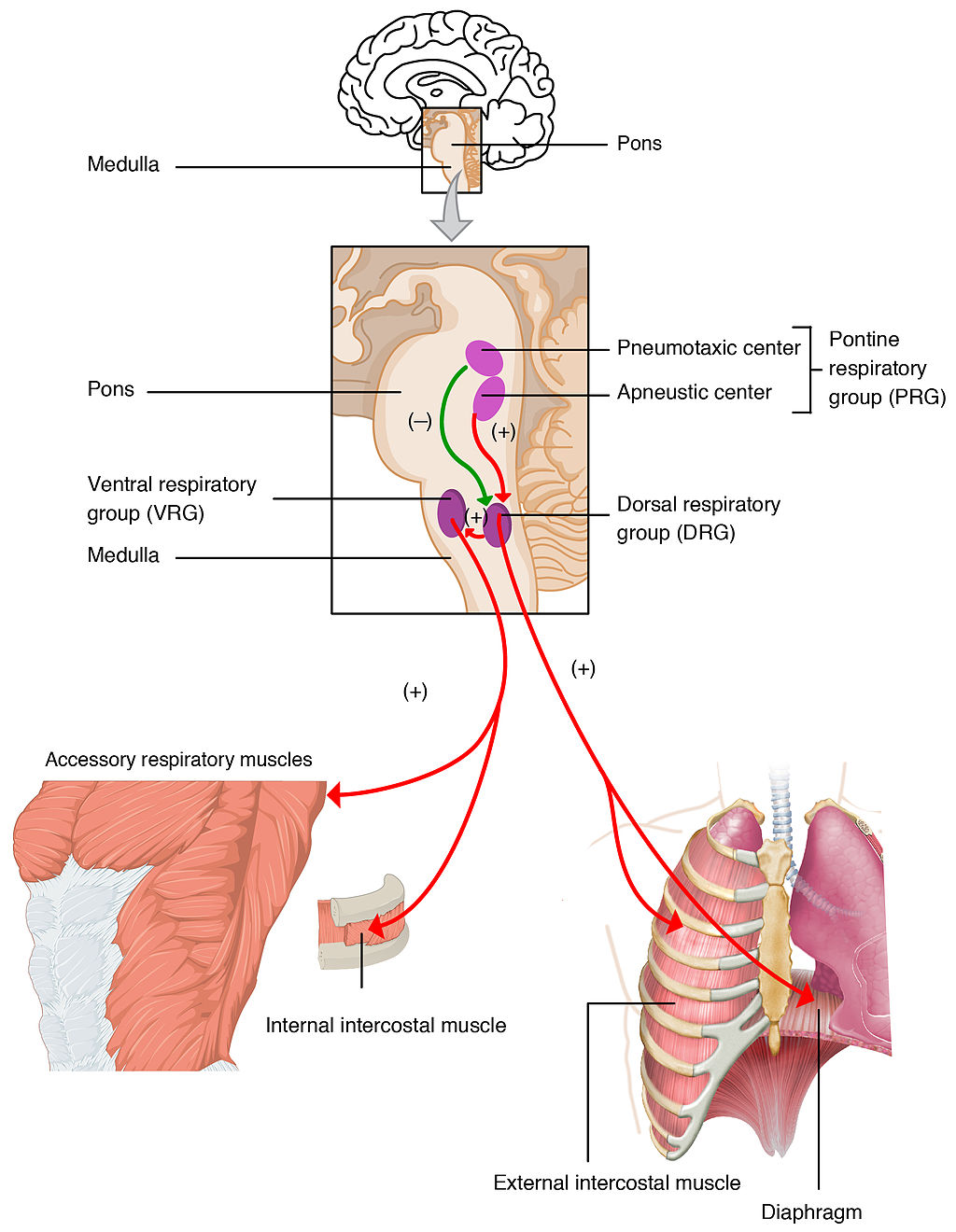
The opposite events occur when the level of carbon dioxide in the blood becomes too low and blood pH rises. This may occur with involuntary hyperventilation, which can happen in panic attacks, episodes of severe pain, asthma attacks, and many other situations. When you hyperventilate, you blow off a lot of carbon dioxide, leading to a drop in blood levels of carbon dioxide. The blood becomes more basic (alkaline), causing its pH to rise.
Nasal vs. Mouth Breathing
Nasal breathing is breathing through the nose rather than the mouth, and it is generally considered to be superior to mouth breathing. The hair-lined nasal passages do a better job of filtering particles out of the air before it moves deeper into the respiratory tract. The nasal passages are also better at warming and moistening the air, so nasal breathing is especially advantageous in the winter when the air is cold and dry. In addition, the smaller diameter of the nasal passages creates greater pressure in the lungs during exhalation. This slows the emptying of the lungs, giving them more time to extract oxygen from the air.
Feature: Myth vs. Reality
Drowning is defined as respiratory impairment from being in or under a liquid. It is further classified according to its outcome into: death, ongoing health problems, or no ongoing health problems (full recovery). Four hundred Canadians die annually from drowning, and drowning is one of the leading causes of death in children under the age of five. There are some potentially dangerous myths about drowning, and knowing what they are might save your life or the life of a loved one, especially a child.
| Myth | Reality |
|---|---|
| "People drown when they aspirate water into their lungs." | Generally, in the early stages of drowning, very little water enters the lungs. A small amount of water entering the trachea causes a muscular spasm in the larynx that seals the airway and prevents the passage of water into the lungs. This spasm is likely to last until unconsciousness occurs. |
| "You can tell when someone is drowning because they will shout for help and wave their arms to attract attention." | The muscular spasm that seals the airway prevents the passage of air, as well as water, so a person who is drowning is unable to shout or call for help. In addition, instinctive reactions that occur in the final minute or so before a drowning person sinks under the water may look similar to calm, safe behavior. The head is likely to be low in the water, tilted back, with the mouth open. The person may have uncontrolled movements of the arms and legs, but they are unlikely to be visible above the water. |
| "It is too late to save a person who is unconscious in the water." | An unconscious person rescued with an airway still sealed from the muscular spasm of the larynx stands a good chance of full recovery if they start receiving CPR within minutes. Without water in the lungs, CPR is much more effective. Even if cardiac arrest has occurred so the heart is no longer beating, there is still a chance of recovery. The longer the brain goes without oxygen, however, the more likely brain cells are to die. Brain death is likely after about six minutes without oxygen, except in exceptional circumstances, such as young people drowning in very cold water. There are examples of children surviving, apparently without lasting ill effects, for as long as an hour in cold water. Rescuers retrieving a child from cold water should attempt resuscitation even after a protracted period of immersion. |
| "If someone is drowning, you should start administering CPR immediately, even before you try to get the person out of the water." | Removing a drowning person from the water is the first priority, because CPR is ineffective in the water. The goal should be to bring the person to stable ground as quickly as possible and then to start CPR. |
| "You are unlikely to drown unless you are in water over your head." | Depending on circumstances, people have drowned in as little as 30 mm (about 1 ½ in.) of water. Inebriated people or those under the influence of drugs, for example, have been known to have drowned in puddles. Hundreds of children have drowned in the water in toilets, bathtubs, basins, showers, pails, and buckets (see Figure 13.3.5). |

13.3 Summary
- Breathing, or ventilation, is the two-step process of drawing air into the lungs (inhaling) and letting air out of the lungs (exhaling). Inhalation is an active process that results mainly from contraction of a muscle called the diaphragm. Exhalation is typically a passive process that occurs mainly due to the elasticity of the lungs when the diaphragm relaxes.
- Breathing is one of the few vital bodily functions that can be controlled consciously, as well as unconsciously. Conscious control of breathing is common in many activities, including swimming and singing. There are limits on the conscious control of breathing, however. If you try to hold your breath, for example, you will soon have an irrepressible urge to breathe.
- Unconscious breathing is controlled by respiratory centers in the medulla and pons of the brainstem. They respond to variations in blood pH by either increasing or decreasing the rate of breathing as needed to return the pH level to the normal range.
- Nasal breathing is generally considered to be superior to mouth breathing because it does a better job of filtering, warming, and moistening incoming air. It also results in slower emptying of the lungs, which allows more oxygen to be extracted from the air.
- Drowning is a major cause of death in Canada, in particular in children under the age of five. It is important to supervise small children when they are playing in, around, or with water.
13.3 Review Questions
- Define breathing.
-
- Give examples of activities in which breathing is consciously controlled.
- Explain how unconscious breathing is controlled.
- Young children sometimes threaten to hold their breath until they get something they want. Why is this an idle threat?
- Why is nasal breathing generally considered superior to mouth breathing?
- Give one example of a situation that would cause blood pH to rise excessively. Explain why this occurs.
13.3 Explore More
https://www.youtube.com/watch?v=Kl4cU9sG_08
How breathing works - Nirvair Kaur, TED-Ed, 2012.
https://www.youtube.com/watch?v=yDtKBXOEsoM
How do ventilators work? - Alex Gendler, TED-Ed, 2020.
https://www.youtube.com/watch?v=XFnGhrC_3Gs&feature=emb_logo
How I held my breath for 17 minutes | David Blaine, TED, 2010.
https://www.youtube.com/watch?v=Vca6DyFqt4c&feature=emb_logo
The Ultimate Relaxation Technique: How To Practice Diaphragmatic Breathing For Beginners, Kai Simon, 2015.
Attributions
Figure 13.3.1
US_Marines_butterfly_stroke by Cpl. Jasper Schwartz from U.S. Marine Corps on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 13.3.2
Inhale Exhale/Breathing cycle by Siyavula Education on Flickr is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/) license.
Figure 13.3.3
Trumpet/ Frenchmen Street [photo] by Morgan Petroski on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 13.3.4
Respiratory_Centers_of_the_Brain by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 13.3.5
Lily & Ava in the Kiddie Pool by mob mob on Flickr is used under a CC BY-NC 2.0 (https://creativecommons.org/licenses/by-nc/2.0/) license.
References
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, June 19). Figure 22.20 Respiratory centers of the brain [digital image]. In Anatomy and Physiology (Section 22.3). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/22-3-the-process-of-breathing
Kai Simon. (2015, January 11). The ultimate relaxation technique: How to practice diaphragmatic breathing for beginners. YouTube. https://www.youtube.com/watch?v=Vca6DyFqt4c&feature=youtu.be
TED. (2010, January 19). How I held my breath for 17 minutes | David Blaine. YouTube. https://www.youtube.com/watch?v=XFnGhrC_3Gs&feature=youtu.be
TED-Ed. (2012, October 4). How breathing works - Nirvair Kaur. YouTube. https://www.youtube.com/watch?v=Kl4cU9sG_08&feature=youtu.be
TED-Ed. (2020, May 21). How do ventilators work? - Alex Gendler. YouTube. https://www.youtube.com/watch?v=yDtKBXOEsoM&feature=youtu.be
The way in which scientists and researchers use a systematic approach to answer questions about the world around us.
Divide and Split
Can you guess what the colourful image in Figure 4.13.1 represents? It shows a eukaryotic cell during the process of cell division. In particular, the image shows the cell in a part of cell division called anaphase, where the DNA is being pulled to opposite ends of the cell. Normally, DNA is located in the nucleus of most human cells. The nucleus divides before the cell itself splits in two, and before the nucleus divides, the cell’s DNA is replicated (or copied). There must be two copies of the DNA so that each daughter cell will have a complete copy of the genetic material from the parent cell. How is the replicated DNA sorted and separated so that each daughter cell gets a complete set of the genetic material? To answer that question, you first need to know more about DNA and the forms it takes.
The Forms of DNA
Except when a eukaryotic cell divides, its nuclear DNA exists as a grainy material called chromatin. Only once a cell is about to divide and its DNA has replicated does DNA condense and coil into the familiar X-shaped form of a chromosome, like the one shown below.
Most cells in the human body have two pairs of 23 different chromosomes, for a total of 46 chromosomes. Cells that have two pairs of chromosomes are called diploid. Because DNA has already replicated when it coils into a chromosome, each chromosome actually consists of two identical structures called sister chromatids. Sister chromatids are joined together at a region called a centromere.
Mitosis
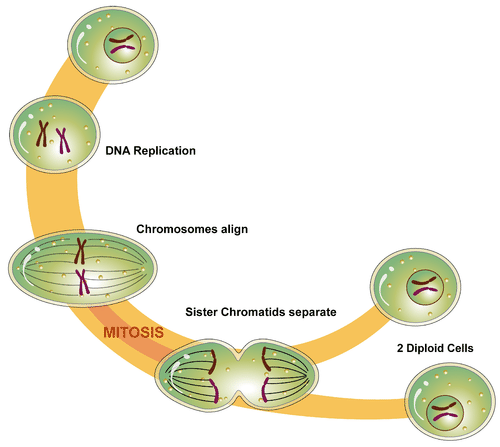
The process in which the nucleus of a eukaryotic cell divides is called mitosis. During mitosis, the two sister chromatids that make up each chromosome separate from each other and move to opposite poles of the cell. This is shown in the figure below.
Mitosis actually occurs in four phases. The phases are called prophase, metaphase, anaphase, and telophase.
Prophase
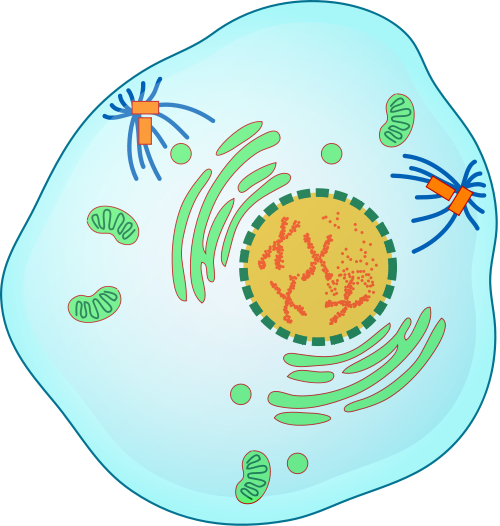
The first and longest phase of mitosis is prophase. During prophase, chromatin condenses into chromosomes, and the nuclear envelope (the membrane surrounding the nucleus) breaks down. In animal cells, the centrioles near the nucleus begin to separate and move to opposite poles of the cell. Centrioles are small organelles found only in eukaryotic cells. They help ensure that the new cells that form after cell division each contain a complete set of chromosomes. As the centrioles move apart, a spindle starts to form between them. The spindle consists of fibres made of microtubules.
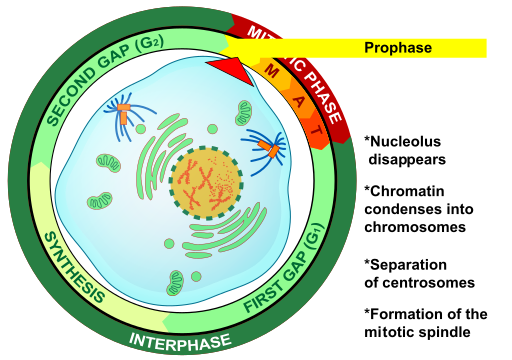
Metaphase
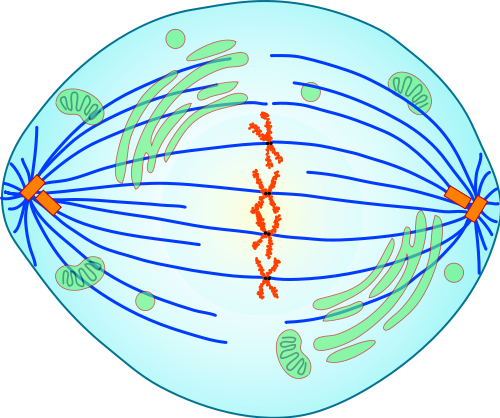
During metaphase, spindle fibres attach to the centromere of each pair of sister chromatids. As you can see in Figure 4.13.7, the sister chromatids line up at the equator (or center) of the cell. The spindle fibres ensure that sister chromatids will separate and go to different daughter cells when the cell divides.
Anaphase
During anaphase, sister chromatids separate and the centromeres divide. The sister chromatids are pulled apart by the shortening of the spindle fibres. This is a little like reeling in a fish by shortening the fishing line. One sister chromatid moves to one pole of the cell, and the other sister chromatid moves to the opposite pole. At the end of anaphase, each pole of the cell has a complete set of chromosomes.
Telophase
During telophase, the chromosomes begin to uncoil and form chromatin. This prepares the genetic material for directing the metabolic activities of the new cells. The spindle also breaks down, and new nuclear envelopes form.
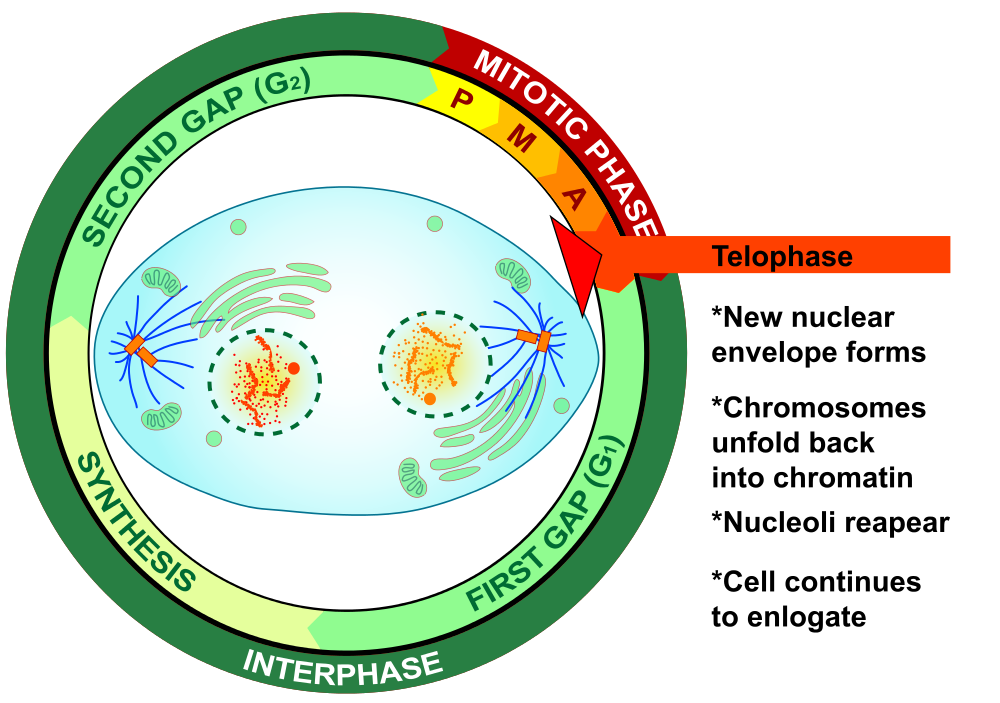
Cytokinesis

Cytokinesis is the final stage of cell division. During cytokinesis, the cytoplasm splits in two and the cell divides, as shown below. In animal cells, the plasma membrane of the parent cell pinches inward along the cell’s equator until two daughter cells form. Thus, the goal of mitosis and cytokinesis is now complete, because one parent cell has given rise to two daughter cells. The daughter cells have the same chromosomes as the parent cell.
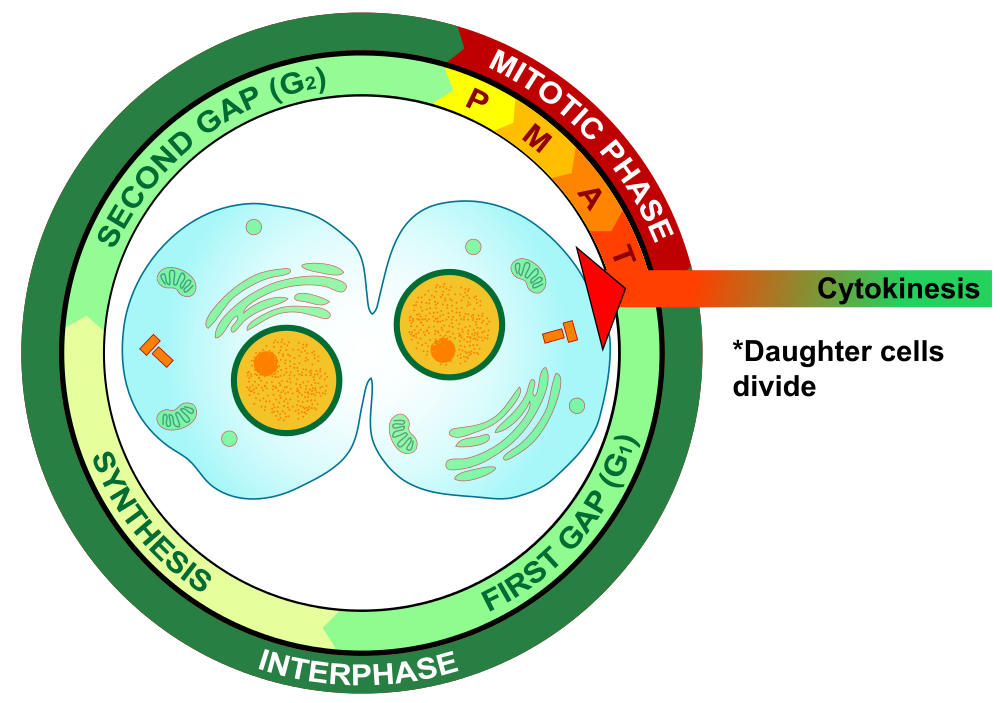
4.13 Summary
- Until a eukaryotic cell divides, its nuclear DNA exists as a grainy material called chromatin. After DNA replicates and the cell is about to divide, the DNA condenses and coils into the X-shaped form of a chromosome. Each chromosome actually consists of two sister chromatids, which are joined together at a centromere.
- Mitosis is the process during which the nucleus of a eukaryotic cell divides. During this process, sister chromatids separate from each other and move to opposite poles of the cell. This happens in four phases: prophase, metaphase, anaphase, and telophase.
- Cytokinesis is the final stage of cell division, during which the cytoplasm splits in two and two daughter cells form.
4.13 Review Questions
- Describe the different forms that DNA takes before and during cell division in a eukaryotic cell.
-
- Identify the four phases of mitosis in an animal cell, and summarize what happens during each phase.
- Order the diagrams of the stages of mitosis:
- Explain what happens during cytokinesis in an animal cell.
- What do you think would happen if the sister chromatids of one of the chromosomes did not separate during mitosis?
- True or False:
4.13 Explore More
https://www.youtube.com/watch?time_continue=3&v=C6hn3sA0ip0&feature=emb_logo
Mitosis, NDSU Virtual Cell Animations project (ndsuvirtualcell), 2012.
https://www.youtube.com/watch?time_continue=19&v=EA0qxhR2oOk&feature=emb_logo
Nondisjunction (Trisomy 21) - An Animated Tutorial, Kristen Koprowski, 2012.
Attributions
Figure 4.13.1
Anaphase_IF by Roy van Heesbeen on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.2
Chromosomes by OpenClipArt-Vectors on Pixabay is used under the Pixabay License (https://pixabay.com/service/license/).
Figure 4.13.3
Chromosome/ Chromatid/ Sister Chromatid by Christine Miller is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.4
Simple Mitosis by Mariana Ruiz Villarreal [LadyofHats] via CK-12 Foundation is used under a CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/) license.
 ©CK-12 Foundation Licensed under
©CK-12 Foundation Licensed under ![]() • Terms of Use • Attribution
• Terms of Use • Attribution
Figure 4.13.5
Mitotic Prophase [tiny] by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.6
Prophase Eukaryotic Mitosis by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.7
Mitotic_Metaphase by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.8
Metaphase Eukaryotic Mitosis by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.9
Anaphase [adapted] by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.10
Anaphase_eukaryotic_mitosis.svg by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.11
Mitotic Telophase by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.12
Telophase Eukaryotic Mitosis by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.13
Mitotic Cytokinesis by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 4.13.14
Cytokinesis Eukaryotic Mitosis by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
References
Koprowski, K., Cabey, R. [Kristen Koprowski]. (2012). Nondisjunction (Trisomy 21) - An Animated Tutorial. YouTube. https://www.youtube.com/watch?v=EA0qxhR2oOk&feature=youtu.be
NDSU Virtual Cell Animations project [ndsuvirtualcell]. (2012). Mitosis. YouTube. https://www.youtube.com/watch?v=C6hn3sA0ip0&t=21s
Acquired Immunodeficiency Syndrome - a chronic, potentially life-threatening condition caused by the human immunodeficiency virus (HIV). By damaging your immune system, HIV interferes with your body's ability to fight infection and disease.
A condition in which you don't have enough healthy red blood cells to carry adequate oxygen to the body's tissues resulting in symptoms including weakness and fatigue.
After reading this chapter, you should be able to see numerous connections between chemistry, human life, and health. In Joseph’s situation, chemistry is involved in the reasons why his father has diabetes, why his personal risk of getting diabetes is high, and why the dietary changes he is considering could be effective.
Type 2 diabetes affects populations worldwide and is caused primarily by a lack of response in the body to the hormone insulin, which causes problems in the regulation of blood sugar, or glucose. Insulin is a peptide hormone, and as you have learned, peptides are chains of amino acids. Therefore, insulin is in the class of biochemical compounds called proteins. Joseph is at increased risk of diabetes partly because there is a genetic component to the disease. DNA, which is a type of chemical compound called a nucleic acid, is passed down from parents to their offspring, and carries the instructions for the production of proteins in units called genes. If there is a problem in a gene (or genes) that contributes to the development of a disease, such as type 2 diabetes, this can get passed down to the offspring and may raise that child’s risk of getting the disease.
But genetics is only part of the reason why Joseph is at an increased risk of diabetes. Obesity itself is a risk factor, and one that can be shared in families due to shared lifestyle factors (such as poor diet and lack of exercise), as well as genetics. Consumption of too many refined carbohydrates (like white bread and soda) may also contribute to obesity and the development of diabetes. As you probably now know, these simple carbohydrates are more easily and quickly broken down in the digestive system into glucose than larger complex carbohydrate molecules, such as those found in vegetables and whole grains. This can lead to dramatic spikes in blood sugar levels, which is particularly problematic for people with diabetes because they have trouble maintaining their blood sugar at a safe level. You can understand why Joseph’s father limits his consumption of refined carbohydrates, and in fact, some scientific studies have shown that avoiding refined carbohydrates may actually help reduce the risk of getting diabetes in the first place.

Joseph’s friend recommended eating a low fat, high carbohydrate diet to lose weight, but you can see that the type of carbohydrate — simple or complex — is an important consideration. Eating a large amount of white bread and rice may not help Joseph reduce his risk of diabetes, but a healthy diet that helps him lose weight may lower his risk of diabetes, since obesity itself is a factor. Which specific diet will work best to help him lose weight probably depends on a variety of factors, including his biology, lifestyle, and food preferences. Joseph should consult with his doctor about his diet and exercise plan, so that his specific situation can be taken into account and monitored by a medical professional.
Drinking enough water is usually good advice for everyone, especially if it replaces sugary drinks like soda. You now know that water is important for many of the chemical reactions that take place in the body. But you can have too much of a good thing — as in the case of marathon runners who can make themselves sick from drinking too much water! As you can see, proper balance, or homeostasis, is very important to the health of living organisms.
Finally, you probably now realize that “chemicals” do not have to be scary, toxic substances. All matter consists of chemicals, including water, your body, and healthy fresh fruits and vegetables, like the ones pictured in Figure 3.12.2. When people advocate “clean eating” and avoiding “chemicals” in food, they are usually referring to avoiding synthetic — or man-made — chemical additives, such as preservatives. This can be a healthy way to eat because it involves eating a variety of whole, fresh, unprocessed foods. But there is no reason to be scared of chemicals in general — they are simply molecules and how they react depends on what they are, what other molecules are present, and the environmental conditions surrounding them.
Chapter 3 Summary
By now, you should have a good understanding of the basics of the chemistry of life. Specifically, you have learned:
- All matter consists of chemical substances. A chemical substance has a definite and consistent composition and may be either an element or a compound.
- An element is a pure substance that cannot be broken down into other types of substances.
- An atom is the smallest particle of an element that still has the properties of that element. Atoms, in turn, are composed of subatomic particles, including negative electrons, positive protons, and neutral neutrons. The number of protons in an atom determines the element it represents.
- Atoms have equal numbers of electrons and protons, so they have no charge. Ions are atoms that have lost or gained electrons, so they have either a positive or negative charge. Atoms with the same number of protons but different numbers of neutrons are called isotopes.
- There are almost 120 known elements. The majority of elements are metals. A smaller number are nonmetals, including carbon, hydrogen, and oxygen.
- A compound is a substance that consists of two or more elements in a unique composition. The smallest particle of a compound is called a molecule. Chemical bonds hold together the atoms of molecules. Compounds can form only in chemical reactions, and they can break down only in other chemical reactions.
- Biochemical compounds are carbon-based compounds found in living things. They make up cells and other structures of organisms and carry out life processes. Most biochemical compounds are large molecules called polymers that consist of many repeating units of smaller molecules called monomers.
- There are millions of different biochemical compounds, but all of them fall into four major classes: carbohydrates, lipids, proteins, and nucleic acids.
- Carbohydrates are the most common class of biochemical compounds. They provide cells with energy, store energy, and make up organic structures, such as the cell walls of plants. The basic building block of carbohydrates is the monosaccharide.
- Sugars are short-chain carbohydrates that supply us with energy. Simple sugars, such as glucose, consist of just one monosaccharide. Some sugars, such as sucrose (or table sugar) consist of two monosaccharides and are called disaccharides.
- Complex carbohydrates, or polysaccharides, consist of hundreds or even thousands of monosaccharides. They include starch, glycogen, cellulose, and chitin.
- Starch is made by plants to store energy and is readily broken down into its component sugars during digestion.
- Glycogen is made by animals and fungi to store energy and plays a critical part in the homeostasis of blood glucose levels in humans.
- Cellulose is the most common biochemical compound in living things. It forms the cell walls of plants and certain algae. Humans cannot digest cellulose, but it makes up most of the crucial dietary fibre in the human diet.
- Chitin makes up organic structures, such as the cell walls of fungi and the exoskeletons of insects and other arthropods.
- Lipids include fats and oils. They store energy, form cell membranes, and carry messages.
- Lipid molecules consist mainly of repeating units called fatty acids. Fatty acids may be saturated or unsaturated, depending on the proportion of hydrogen atoms they contain. Animals store fat as saturated fatty acids, while plants store fat as unsaturated fatty acids.
- Types of lipids include triglycerides, phospholipids, and steroids.
- Triglycerides contain glycerol (an alcohol) in addition to fatty acids. Humans and other animals store fat as triglycerides in fat cells.
- Phospholipids contain phosphate and glycerol in addition to fatty acids. They are the main component of cell membranes in all living things.
- Steroids are lipids with a four-ring structure. Some steroids, such as cholesterol, are important components of cell membranes. Many other steroids are hormones.
- In living things, proteins include enzymes, antibodies, and numerous other important compounds. They help cells keep their shape, make up muscles, speed up chemical reactions, and carry messages and materials (among other functions).
- Proteins are made up of small monomer molecules called amino acids.
- Long chains of amino acids form polypeptides. The sequence of amino acids in polypeptides makes up the primary structure of proteins. Secondary structure refers to configurations such as helices and sheets within polypeptide chains. Tertiary structure is a protein's overall three-dimensional shape, which controls the molecule's basic function. A quaternary structure forms if multiple protein molecules join together and function as a complex.
- The chief characteristic that allows proteins' diverse functions is their ability to bind specifically and tightly with other molecules.
- Nucleic acids include DNA and RNA. They encode instructions for making proteins, helping make proteins, and passing the encoded instructions from parents to offspring.
- Nucleic acids are built of monomers called nucleotides, which bind together in long chains to form polynucleotides. DNA consists of two polynucleotides, and RNA consists of one polynucleotide.
- Each nucleotide consists of a sugar molecule, phosphate group, and nitrogen base. Sugars and phosphate groups of adjacent nucleotides bind together to form the "backbone" of the polynucleotide. Bonds between complementary bases hold together the two polynucleotide chains of DNA and cause it to take on its characteristic double helix shape.
- DNA makes up genes, and the sequence of nitrogen bases in DNA makes up the genetic code for the synthesis of proteins. RNA helps synthesize proteins in cells. The genetic code in DNA is also passed from parents to offspring during reproduction, explaining how inherited characteristics are passed from one generation to the next.
- A chemical reaction is a process that changes some chemical substances into others. A substance that starts a chemical reaction is called a reactant, and a substance that forms in a chemical reaction is called a product. During the chemical reaction, bonds break in reactants and new bonds form in products.
- Chemical reactions can be represented by chemical equations. According to the law of conservation of mass, mass is always conserved in a chemical reaction, so a chemical equation must be balanced, with the same number of atoms of each type of element in the products as in the reactants.
- Many chemical reactions occur all around us each day, such as iron rusting and organic matter rotting, but not all changes are chemical processes. Some changes, such as ice melting or paper being torn into smaller pieces, are physical processes that do not involve chemical reactions and the formation of new substances.
- All chemical reactions involve energy, and they require activation energy to begin. Exothermic reactions release energy. Endothermic reactions absorb energy.
- Biochemical reactions are chemical reactions that take place inside living things. The sum of all the biochemical reactions in an organism is called metabolism. Metabolism includes catabolic reactions (exothermic reactions) and anabolic reactions (endothermic reactions).
- Most biochemical reactions require a biological catalyst called an enzyme to speed up the reaction by reducing the amount of activation energy needed for the reaction to begin. Most enzymes are proteins that affect just one specific substance, called the enzyme's substrate.
- Virtually all living things on Earth require liquid water. Only a tiny per cent of Earth's water is fresh liquid water. Water exists as a liquid over a wide range of temperatures, and it dissolves many substances. These properties depend on water's polarity, which causes water molecules to "stick" together through weak bonds called hydrogen bonds.
- The human body is about 70 per cent water (outside of fat). Organisms need water to dissolve many substances and for most biochemical processes, including photosynthesis and cellular respiration.
- A solution is a mixture of two or more substances that has the same composition throughout. Many solutions consist of water and one or more dissolved substances.
- Acidity is a measure of the hydronium ion concentration in a solution. Pure water has a very low concentration and a pH of 7, which is the point of neutrality on the pH scale. Acids have a higher hydronium ion concentration than pure water and a pH lower than 7. Bases have a lower hydronium ion concentration than pure water and a pH higher than 7.
- Many acids and bases in living things are secreted to provide the proper pH for enzymes to work properly.
Now you understand the chemistry of the molecules that make up living things. In the next chapter, you will learn how these molecules make up the basic unit of structure and function in living organisms — cells — and you will be able to understand some of the crucial chemical reactions that occur within cells.
Chapter 3 Review
-
- The chemical formula for the complex carbohydrate glycogen is C24H42O21.
- What are the elements in glycogen?
- How many atoms are in one molecule of glycogen?
- Is glycogen an ion? Why or why not?
- Is glycogen a monosaccharide or a polysaccharide? Besides memorizing this fact, how would you know this based on the information in the question?
- What is the function of glycogen in the human body?
- What is the difference between an ion and a polar molecule? Give an example of each in your explanation.
- Define monomer and polymer.
-
- What is the difference between a protein and a polypeptide?
-
- People with diabetes have trouble controlling the level of glucose in their bloodstream. Knowing this, why do you think it is often recommended that people with diabetes limit their consumption of carbohydrates?
- Identify each of the following reactions as endothermic or exothermic.
- cellular respiration
- photosynthesis
- catabolic reactions
- anabolic reactions
- Pepsin is an enzyme in the stomach that helps us digest protein. Answer the following questions about pepsin:
- What is the substrate for pepsin?
- How does pepsin work to speed up protein digestion?
- Given what you know about the structure of proteins, what do you think are some of the products of the reaction that pepsin catalyzes?
- The stomach is normally acidic. What do you think would happen to the activity of pepsin and protein digestion if the pH is raised significantly?
Attributions
Figure 3.13.1
Prevalence_of_Diabetes_by_Percent_of_Country_Population_(2014)_Gradient_Map by Walter Scott Wilkens [Wwilken2], University of Illinois - Urbana Champaign Department of Geography and GIScience, on Wikimedia Commons, is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
Figure 3.13.2
Healthy plate by Melinda Young Stuart on Flickr is used under a CC BY-NC-ND 2.0 (https://creativecommons.org/licenses/by-nc-nd/2.0/) license.
The process by which information from a gene is used in the synthesis of a functional protein.

