14.2 Introduction to the Cardiovascular System
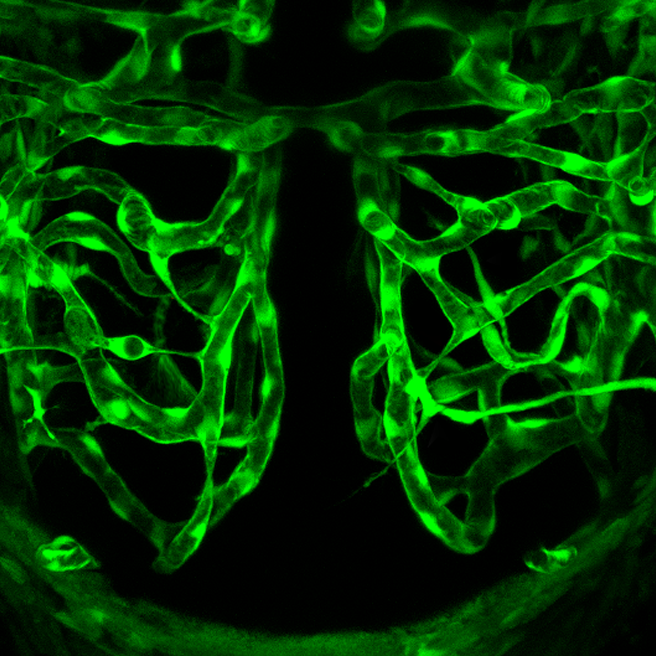
Ant Hill or Plumbing System?
What do you think the picture in Figure 14.2.1 shows? Is it a maze of underground passageways in an ant hill? A network of interconnected pipes in a complex plumbing system? The picture actually shows something that, like ant tunnels and plumbing pipes, functions as a transportation system. It shows a network of blood vessels, which are part of the cardiovascular system.
What is the Cardiovascular System?
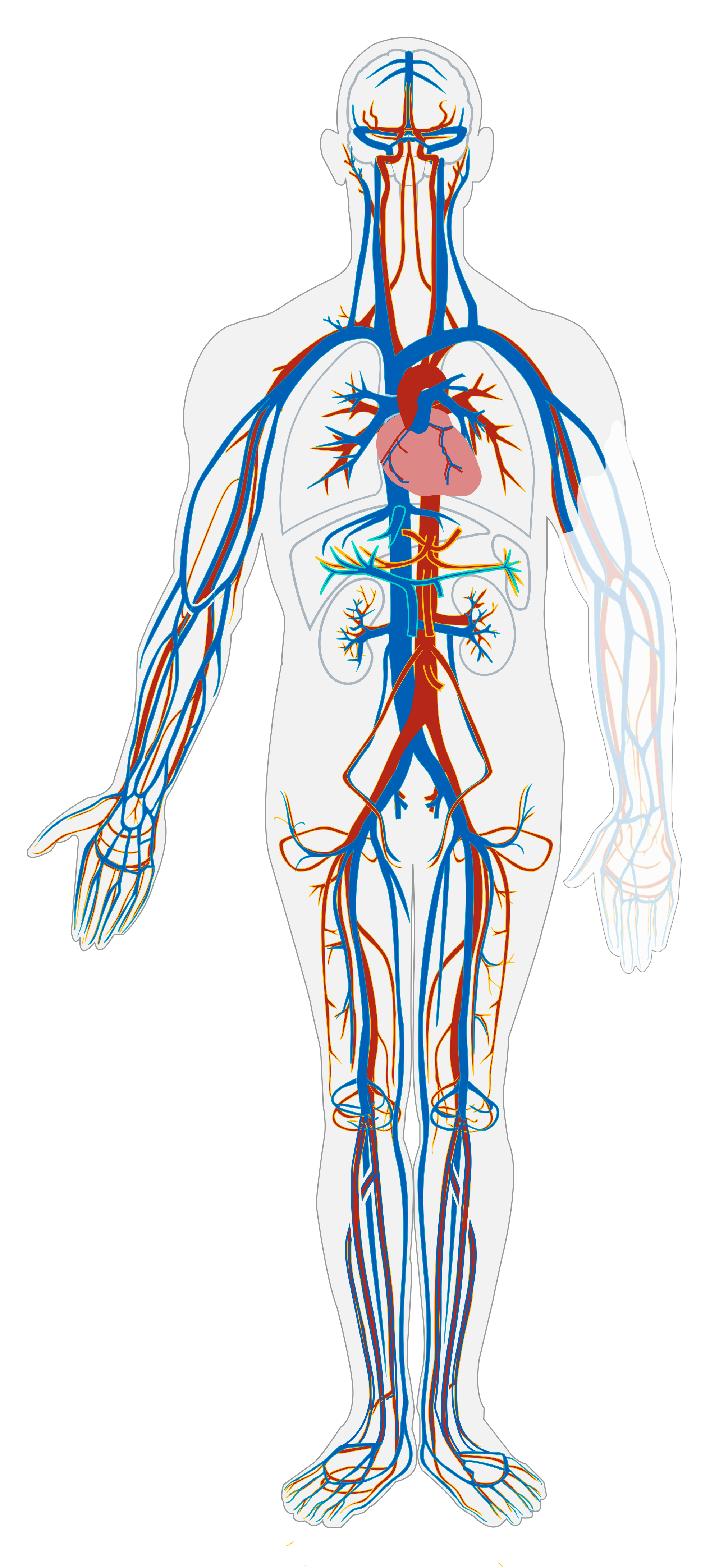
The cardiovascular system, also called the circulatory system, is the organ system that transports materials to and from all the cells of the body. The materials carried by the cardiovascular system include oxygen from the lungs, nutrients from the digestive system, hormones from glands of the endocrine system, and waste materials from cells throughout the body. Transport of these and many other materials is necessary to maintain homeostasis of the body. The main components of the cardiovascular system are the heart, blood vessels, and blood. Each of these components is shown in Figure 14.2.2 and introduced below.
Heart
The heart is a muscular organ in the chest. It consists mainly of cardiac muscle tissue, and it pumps blood through blood vessels by repeated, rhythmic contractions. As shown in Figure 14.2.3, the heart has four inner chambers: a right atrium and ventricle, and a left atrium and ventricle. On each side of the heart, blood is pumped from the atrium to the ventricle below it, and from the ventricle out of the heart. The heart also contains several valves that allow blood to flow only in the proper direction through the heart.
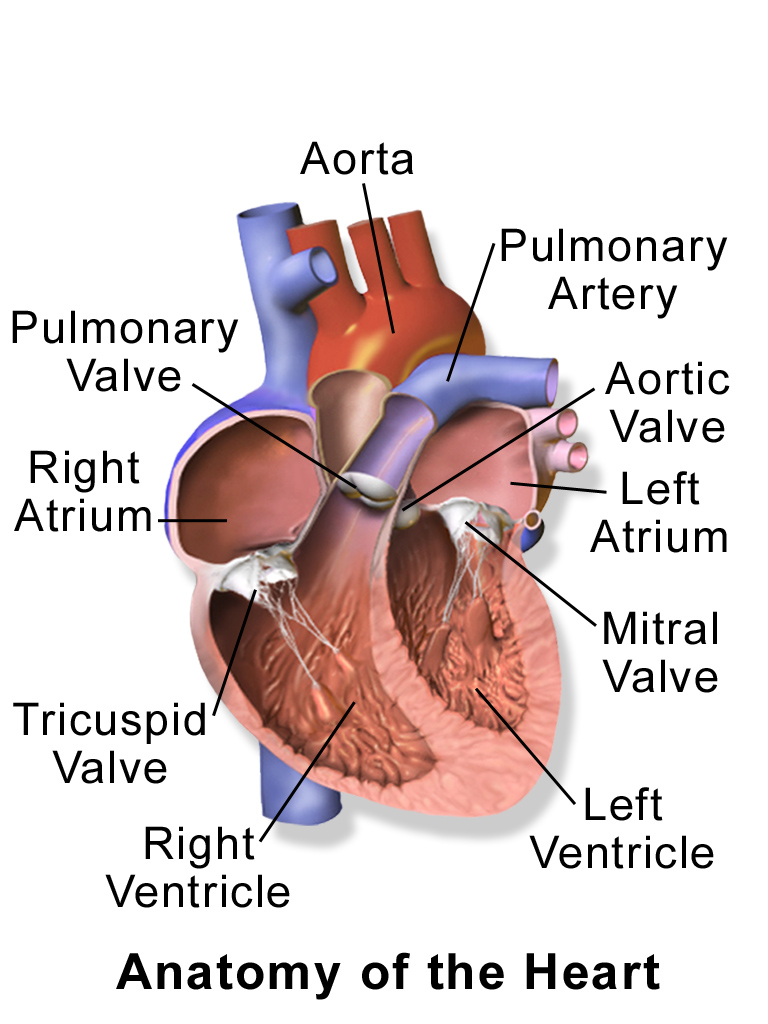
As you may have noticed, the Figure 14.2.3 diagram labels the right side of the heart on the left side of the diagram, and vice versa. This is because it is assumed that in this diagram, the heart appears as if the patient was facing us – the patient’s left side is on our right side!
Unlike skeletal muscle, cardiac muscle routinely contracts without stimulation by the nervous system. Specialized cardiac muscle cells send out electrical impulses that stimulate the contractions. As a result, the atria and ventricles normally contract with just the right timing to keep blood pumping efficiently through the heart.
Blood Vessels
The blood vessels of the cardiovascular system are like a network of interconnected, one-way roads that range from superhighways to back alleys. Like a network of roads, the blood vessels are tasked with allowing the transport of materials from one place to another. There are three major types of blood vessels: arteries, veins, and capillaries. They are illustrated in Figure 14.2.4 and described below.
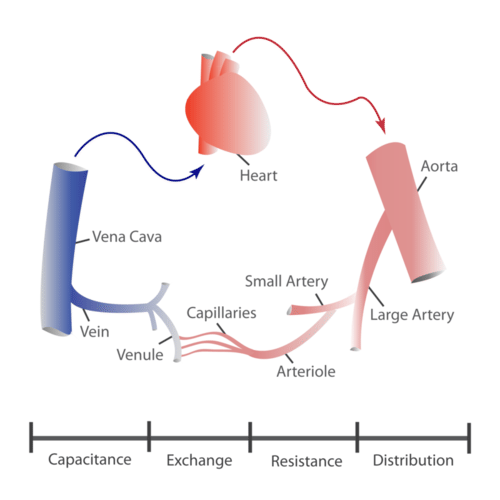
- Arteries are blood vessels that carry blood away from the heart (except for the arteries that actually supply blood to the heart muscle). Most arteries carry oxygen-rich blood, and one of their main functions is distributing oxygen to tissues throughout the body. The smallest arteries are called arterioles.
- Veins are blood vessels that carry blood toward the heart. Most veins carry deoxygenated blood. The smallest veins are called venules.
- Capillaries are the smallest blood vessels, and they connect arterioles and venules. As they pass through tissues, they exchange substances (including oxygen) with cells.
Two Circulations
Cells throughout the body need a constant supply of oxygen. They get oxygen from capillaries in the systemic circulation. The systemic circulation is just one of two interconnected circulations that make up the human cardiovascular system. The other circulation is the pulmonary system, which is where blood picks up oxygen to carry to cells. It takes blood about 20 seconds to make one complete transit through both circulations (see Figure 14.2.5).
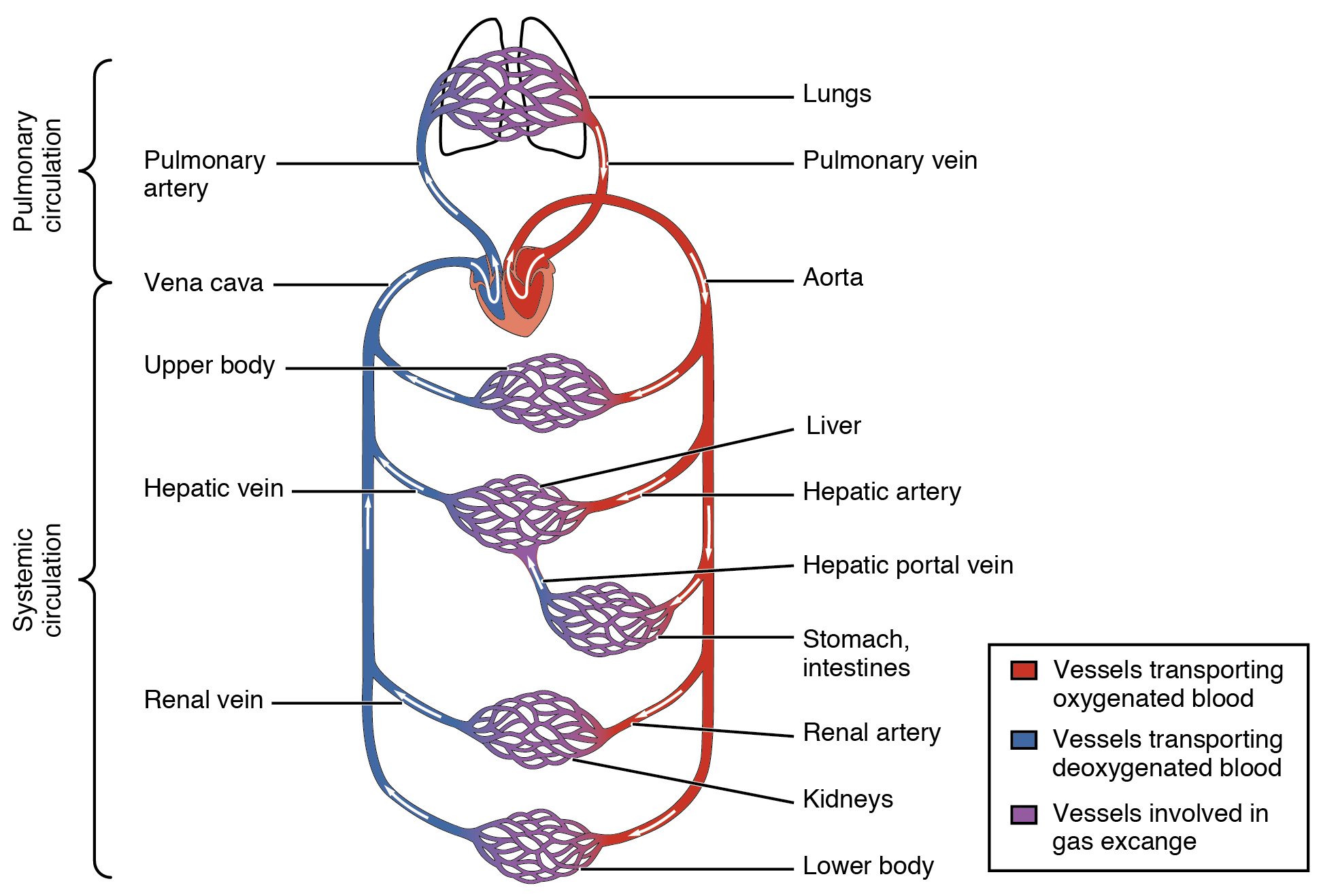
Pulmonary Circuit
The pulmonary circuit involves only the heart, the lungs, and the major blood vessels that connect them (illustrated in Figure 14.2.6). Blood moves through the pulmonary circuit from the heart, to the lungs, and then back to the heart again, becoming oxygenated in the process. Specifically, the right ventricle of the heart pumps deoxygenated blood into the right and left pulmonary arteries. These arteries carry the blood to the right and left lungs, respectively. Oxygenated blood then returns from the right and left lungs through the two right and two left pulmonary veins. All four pulmonary veins enter the left atrium of the heart.
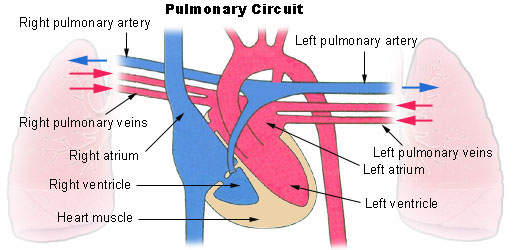
What happens to the blood while it is in the lungs? It passes through increasingly smaller arteries, and finally through capillary networks surrounding the alveoli (see Figure 14.2.7). This is where gas exchange takes place. The deoxygenated blood in the capillaries picks up oxygen from the alveoli, and gives up carbon dioxide to the alveoli. As a result, the blood returning to the heart in the pulmonary veins is almost completely saturated with oxygen.
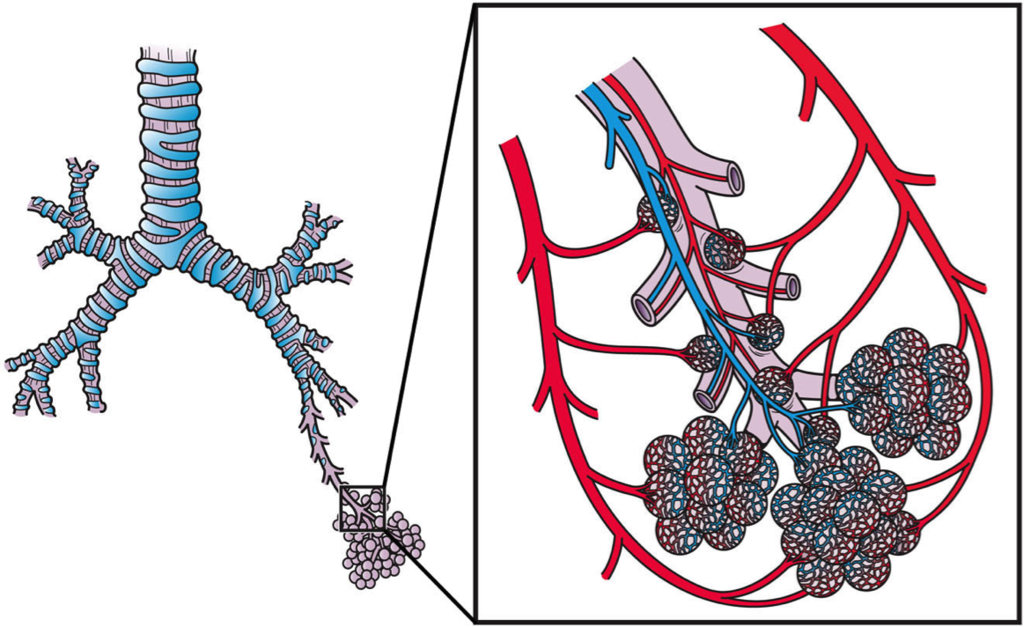
Systemic Circulation
The oxygenated blood that enters the left atrium of the heart in the pulmonary circulation then passes into the systemic circuit. This is the part of the cardiovascular system that transports blood to and from all of the tissues of the body to provide oxygen and nutrients, and to pick up wastes. It consists of the heart and blood vessels that supply the metabolic needs of all the cells in the body, including those of the heart and lungs.
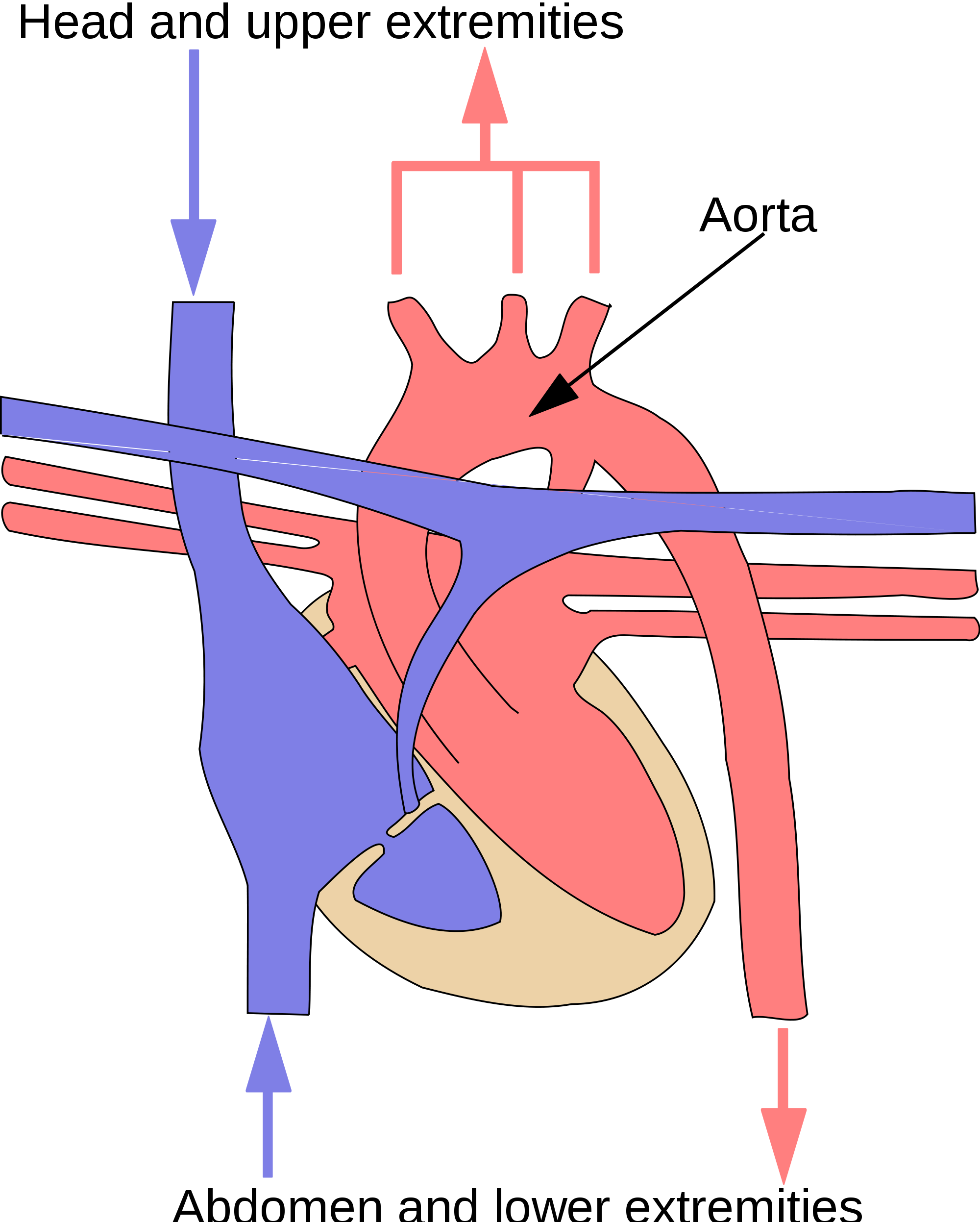
As shown in Figure 14.2.8, in the systemic circulation, the left atrium pumps oxygenated blood to the left ventricle, which pumps the blood directly into the aorta, the body’s largest artery. Major arteries branching off the aorta carry the blood to the head and upper extremities. The aorta continues down through the abdomen and carries blood to the abdomen and lower extremities. The blood then returns to the heart through the network of increasingly larger veins of the systemic circulation. All of the returning blood eventually collects in the superior vena cava (upper body) and inferior vena cava (lower body), which empty directly into the right atrium of the heart.
Blood
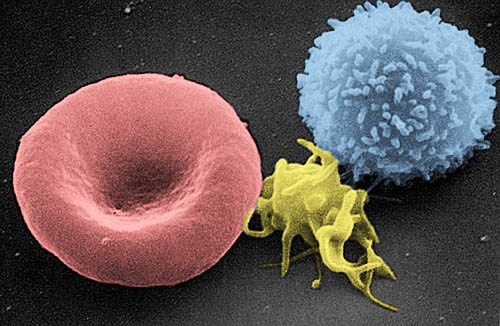
Blood is a fluid connective tissue that circulates throughout the body in blood vessels by the pumping action of the heart. Blood carries oxygen and nutrients to all the body’s cells, and it carries carbon dioxide and other wastes away from the cells to be excreted. Blood also transports many other substances, defends the body against infection, repairs body tissues, and controls the body’s pH, among other functions.
The fluid part of blood is called plasma. It is a yellowish, watery liquid that contains many dissolved substances and blood cells. Types of blood cells in plasma include red blood cells, white blood cells, and platelets, all of which are illustrated in the photomicrograph (Figure 14.2.9) and described below.
- Erythrocytes (red blood cells) have the main function of carrying oxygen in the blood. Red blood cells consist mostly of hemoglobin, a protein containing iron that binds with oxygen.
- Leukocytes (white blood cells) are far fewer in number than red blood cells. They defend the body in various ways. White blood cells called phagocytes, for example, swallow and destroy pathogens, dead cells, and other debris in the blood.
- Thrombocytes (platelets) are cell fragments involved in blood clotting. They stick to tears in blood vessels and to each other, forming a plug at the site of injury. They also release chemicals that are needed for clotting to occur.
14.2 Summary
- The cardiovascular system is the organ system that transports materials to and from all the cells of the body. The main components of the cardiovascular system are the heart, blood vessels, and blood.
- The heart is a muscular organ in the chest that consists mainly of cardiac muscle and pumps blood through blood vessels by repeated, rhythmic contractions. The heart has four chambers through which blood flows, and valves that keep blood flowing in just one direction.
- Blood vessels carry blood throughout the body. Major types of blood vessels are arteries (which mainly carry blood away from the heart), veins (which carry blood toward the heart), and capillaries (which exchange substances between the blood and cells of the body).
- The cardiovascular system has two interconnected circulations. The pulmonary circuit carries blood between the heart and lungs, where blood is oxygenated. The systemic circuit carries blood between the heart and the rest of the body, where it delivers oxygen.
- Blood is a fluid connective tissue that circulates throughout the body in blood vessels. It consists of a liquid part — called plasma — which contains many dissolved substances, and cells, including erythrocytes, leukocytes and thrombocytes.
14.2 Review Questions
- Describe the heart and how it functions.
- Compare and contrast the pulmonary and systemic circulations.
-
- What is blood? What are its chief constituents?
- Name three different types of substances transported by the cardiovascular system.
- Explain why the heart and lungs need blood from the systemic circulation.
- Do blood vessels carrying deoxygenated blood from the body back to the heart get increasingly larger or smaller?
14.2 Explore More
How the heart actually pumps blood – Edmond Hui, TED-Ed, 2014.
Circulatory & Respiratory Systems – CrashCourse Biology #27, CrashCourse, 2012.
The Heart and Circulatory System – How They Work, Mayo Clinic, 2013.
Attributions
Figure 14.2.1
Brain vascular formation [photo] by Liulin Du/ Chen (The National Cancer Institute at Frederick) on PLOS Biology is used under a CC BY 4.0 license.
Figure 14.2.2
Circulatory_System_no_tags.svg by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 14.2.3
Blausen_0462_HeartAnatomy by BruceBlaus on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)
Figure 14.2.4
Structure and functions of the different types of blood vessels by CK-12 Foundation is used under a CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/) license.
Figure 14.2.5
2101_Blood_Flow_Through_the_Heart by OpenStax College on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 14.2.6
Illu_pulmonary_circuit by Arcadian from National Cancer Institute/ SEER Training on Wikimedia Commons is in the the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 14.2.7
Pulmonary_Blood_Circulation by Artwork by Holly Fischer from Open Michigan (Respiratory Tact Slide 20) on Wikimedia Commons is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 14.2.8
systemic_circuit.svg by Surachit on Wikimedia Commons is used under a CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0/) license. (Derivative work based on SEER Training by NCI/ U.S. Government).
Figure 14.2.9
Red_White_Blood_cells by Electron Microscopy Facility at The National Cancer Institute at Frederick (NCI-Frederick) on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
References
Blausen.com Staff. (2014). Medical gallery of Blausen Medical 2014. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436
Betts, J. G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, June 19). Figure 20.2 Cardiovascular circulation [digital image]. In Anatomy and Physiology (Section 7.3). OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/20-1-structure-and-function-of-blood-vessels
Brainard, J/ CK-12 Foundation. (2016). Figure 4 Diagram represents the structure and functions of the different types of blood vessels in the cardiovascular system [digital image]. In CK-12 College Human Biology (Section 16.2) [online Flexbook]. CK12.org. https://www.ck12.org/book/ck-12-college-human-biology/section/16.2/
CrashCourse. (2012, July 30). Circulatory & respiratory systems – CrashCourse Biology #27. YouTube. https://www.youtube.com/watch?v=9fxm85Fy4sQ&feature=youtu.be
Du, J. (2012, August). Brain vasculature formation [digital image]. PLoS Biology, 10(8): ev10.i08. https://doi.org/10.1371/image.pbio.v10.i08 © Chen.
Mayo Clinic. (2013). The heart and circulatory system – How they work. YouTube. https://www.youtube.com/watch?v=CWFyxn0qDEU&t=1s
TED-Ed. (2014, May 20). How the heart actually pumps blood – Edmond Hui. YouTube. https://www.youtube.com/watch?v=ruM4Xxhx32U&feature=youtu.be
Refers to the body system consisting of the heart, blood vessels and the blood. Blood contains oxygen and other nutrients which your body needs to survive. The body takes these essential nutrients from the blood.
Created by CK-12 Foundation/Adapted by Christine Miller

Oxygen Bar
Belly up to the bar and get your favorite... oxygen? That’s right — in some cities, you can get a shot of pure oxygen, with or without your choice of added flavors. Bar patrons inhale oxygen through a plastic tube inserted into their nostrils, paying up to a dollar per minute to inhale the pure gas. Proponents of the practice claim that breathing in extra oxygen will remove toxins from the body, strengthen the immune system, enhance concentration and alertness, increase energy, and even cure cancer! These claims, however, have not been substantiated by controlled scientific studies. Normally, blood leaving the lungs is almost completely saturated with oxygen, even without the use of extra oxygen, so it’s unlikely that a higher concentration of oxygen in air inside the lungs would lead to significantly greater oxygenation of the blood. Oxygen enters the blood in the lungs as part of the process of gas exchange.
What is Gas Exchange?
Gas exchange is the biological process through which gases are transferred across cell membranes to either enter or leave the blood. Oxygen is constantly needed by cells for aerobic cellular respiration, and the same process continually produces carbon dioxide as a waste product. Gas exchange takes place between the blood and cells throughout the body, with oxygen leaving the blood and entering the cells, and carbon dioxide leaving the cells and entering the blood. Gas exchange also takes place between the blood and the air in the lungs, with oxygen entering the blood from the inhaled air inside the lungs, and carbon dioxide leaving the blood and entering the air to be exhaled from the lungs.
Gas Exchange in the Lungs
Alveoli are the basic functional units of the lungs where gas exchange takes place between the air and the blood. Alveoli (singular, alveolus) are tiny air sacs that consist of connective and epithelial tissues. The connective tissue includes elastic fibres that allow alveoli to stretch and expand as they fill with air during inhalation. During exhalation, the fibres allow the alveoli to spring back and expel the air. Special cells in the walls of the alveoli secrete a film of fatty substances called surfactant. This substance prevents the alveolar walls from collapsing and sticking together when air is expelled. Other cells in alveoli include macrophages, which are mobile scavengers that engulf and destroy foreign particles that manage to reach the lungs in inhaled air.
As shown in Figure 13.4.2, alveoli are arranged in groups like clusters of grapes. Each alveolus is covered with epithelium that is just one cell thick. It is surrounded by a bed of pulmonary capillaries, each of which has a wall of epithelium just one cell thick. As a result, gases must cross through only two cells to pass between an alveolus and its surrounding capillaries.
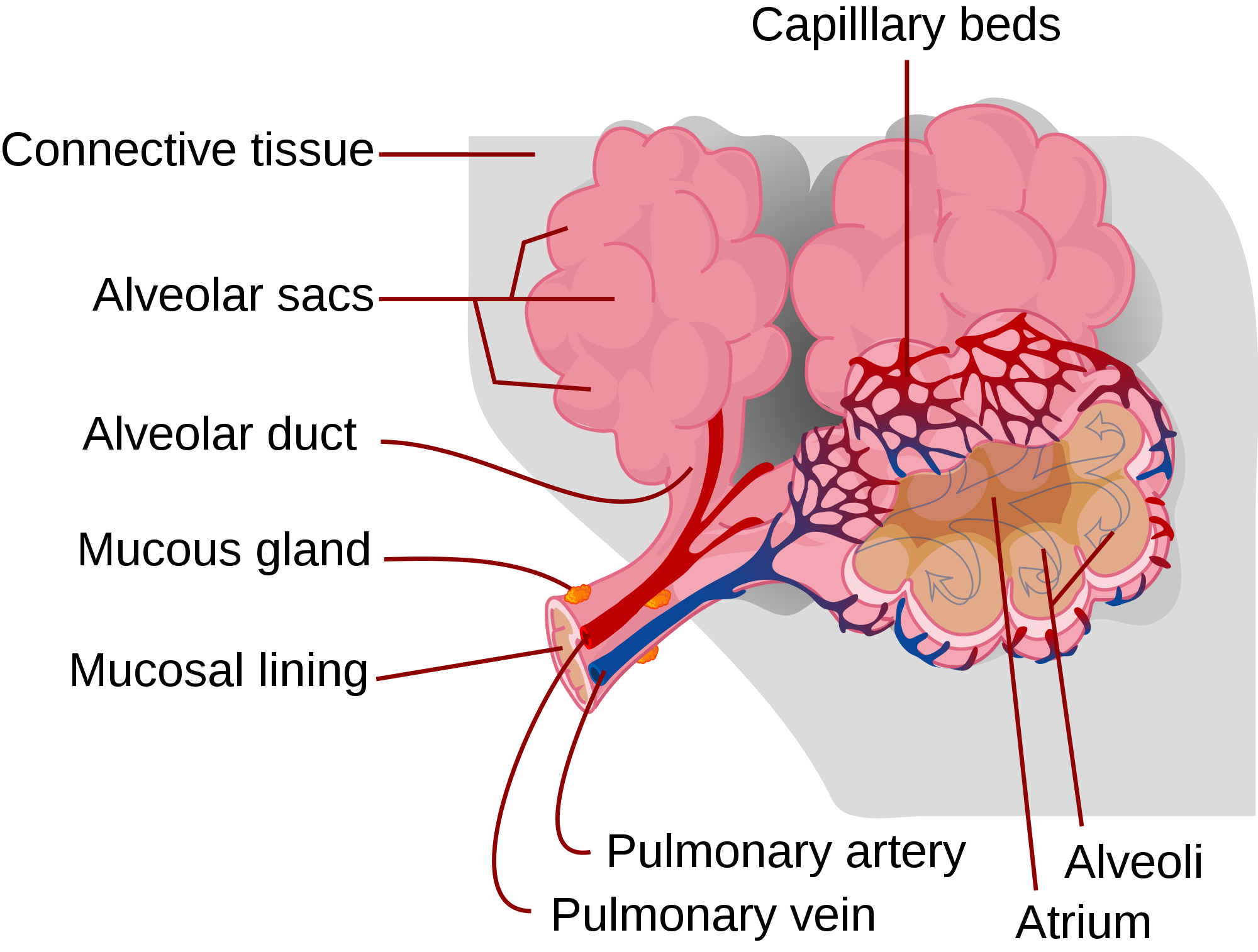
The pulmonary artery (also shown in Figure 13.4.2) carries deoxygenated blood from the heart to the lungs. Then, the blood travels through the pulmonary capillary beds, where it picks up oxygen and releases carbon dioxide. The oxygenated blood then leaves the lungs and travels back to the heart through pulmonary veins. There are four pulmonary veins (two for each lung), and all four carry oxygenated blood to the heart. From the heart, the oxygenated blood is then pumped to cells throughout the body.
Mechanism of Gas Exchange
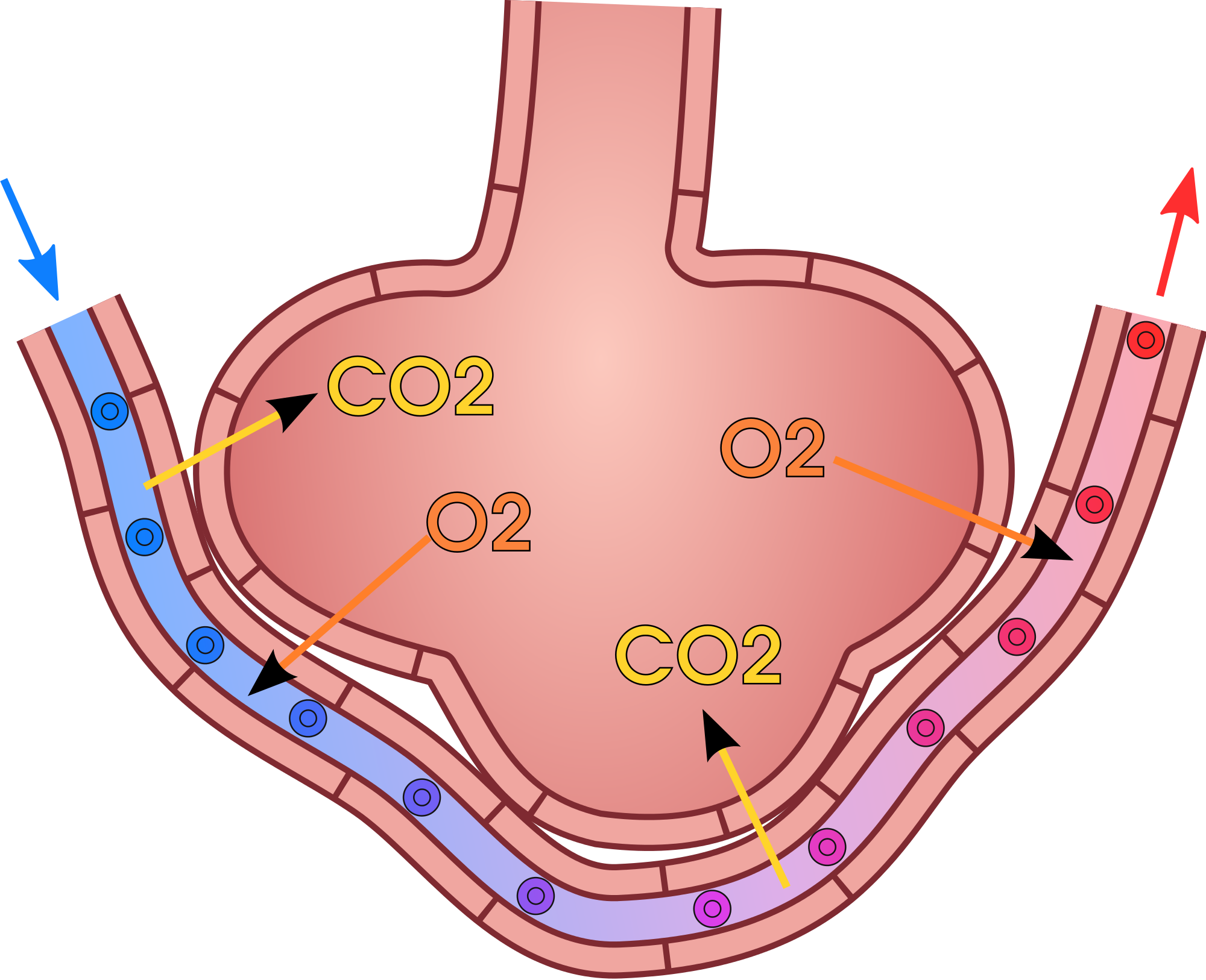
Gas exchange occurs by diffusion across cell membranes. Gas molecules naturally move down a concentration gradient from an area of higher concentration to an area of lower concentration. This is a passive process that requires no energy. To diffuse across cell membranes, gases must first be dissolved in a liquid. Oxygen and carbon dioxide are transported around the body dissolved in blood. Both gases bind to the protein hemoglobin in red blood cells, although oxygen does so more effectively than carbon dioxide. Some carbon dioxide also dissolves in blood plasma.
As shown in Figure 13.4.3, oxygen in inhaled air diffuses into a pulmonary capillary from the alveolus. Carbon dioxide in the blood diffuses in the opposite direction. The carbon dioxide can then be exhaled from the body.
Gas exchange by diffusion depends on having a large surface area through which gases can pass. Although each alveolus is tiny, there are hundreds of millions of them in the lungs of a healthy adult, so the total surface area for gas exchange is huge. It is estimated that this surface area may be as great as 100 m2 (or approximately 1,076 ft²). Often we think of lungs as balloons, but this type of structure would have very limited surface area and there wouldn't be enough space for blood to interface with the air in the alveoli. The structure alveoli take in the lungs is more like a giant mass of soap bubbles — millions of tiny little chambers making up one large mass — this is what increases surface area giving blood lots of space to come into close enough contact to exchange gases by diffusion.
Gas exchange by diffusion also depends on maintaining a steep concentration gradient for oxygen and carbon dioxide. Continuous blood flow in the capillaries and constant breathing maintain this gradient.
- Each time you inhale, there is a greater concentration of oxygen in the air in the alveoli than there is in the blood in the pulmonary capillaries. As a result, oxygen diffuses from the air inside the alveoli into the blood in the capillaries. Carbon dioxide, in contrast, is more concentrated in the blood in the pulmonary capillaries than it is in the air inside the alveoli. As a result, carbon dioxide diffuses in the opposite direction.
- The cells of the body have a much lower concentration of oxygen than does the oxygenated blood that reaches them in peripheral capillaries, which are the capillaries that supply tissues throughout the body. As a result, oxygen diffuses from the peripheral capillaries into body cells. The opposite is true of carbon dioxide. It has a much higher concentration in body cells than it does in the blood of the peripheral capillaries. Thus, carbon dioxide diffuses from body cells into the peripheral capillaries.
13.4 Summary
- Gas exchange is the biological process through which gases are transferred across cell membranes to either enter or leave the blood. Gas exchange takes place continuously between the blood and cells throughout the body, and also between the blood and the air inside the lungs.
- Gas exchange in the lungs takes place in alveoli, which are tiny air sacs surrounded by networks of capillaries. The pulmonary artery carries deoxygenated blood from the heart to the lungs, where it travels through pulmonary capillaries, picking up oxygen and releasing carbon dioxide. The oxygenated blood then leaves the lungs through pulmonary veins.
- Gas exchange occurs by diffusion across cell membranes. Gas molecules naturally move down a concentration gradient from an area of higher concentration to an area of lower concentration. This is a passive process that requires no energy.
- Gas exchange by diffusion depends on the large surface area provided by the hundreds of millions of alveoli in the lungs. It also depends on a steep concentration gradient for oxygen and carbon dioxide. This gradient is maintained by continuous blood flow and constant breathing.
13.4 Review Questions
- What is gas exchange?
- Summarize the flow of blood into and out of the lungs for gas exchange.
-
- Describe the mechanism by which gas exchange takes place.
- Identify the two main factors upon which gas exchange by diffusion depends.
- If the concentration of oxygen were higher inside of a cell than outside of it, which way would the oxygen flow? Explain your answer.
- Why is it important that the walls of the alveoli are only one cell thick?
13.4 Explore More
https://www.youtube.com/watch?v=nRpwdwm06Ic&feature=emb_logo
Oxygen movement from alveoli to capillaries | NCLEX-RN | Khan Academy, khanacademymedicine, 2013.
https://www.youtube.com/watch?v=KmgIqVwytwA&feature=emb_logo
About Carbon Monoxide and Carbon Monoxide Poisoning, EMDPrepare, 2009.
https://www.youtube.com/watch?v=GVU_zANtroE
Oxygen’s surprisingly complex journey through your body - Enda Butler, TED-Ed, 2017.
Attributions
Figure 13.4.1
Oxygen Bar by Farrukh on Flickr is used under a CC BY-NC 2.0 (https://creativecommons.org/licenses/by-nc/2.0/) license.
Figure 13.4.2
Alveolus_diagram.svg by Mariana Ruiz Villarreal [LadyofHats] on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 13.4.3
Gas_exchange_in_the_aveolus.svg by domdomegg on Wikimedia Commons is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
References
EMDPrepare. (2009, December 21). About carbon monoxide and carbon monoxide poisoning. YouTube. https://www.youtube.com/watch?v=KmgIqVwytwA&feature=youtu.be
khanacademymedicine. (2013, February 25). Oxygen movement from alveoli to capillaries | NCLEX-RN | Khan Academy. YouTube. https://www.youtube.com/watch?v=nRpwdwm06Ic&feature=youtu.be
TED-Ed. (2017, April 13). Oxygen’s surprisingly complex journey through your body - Enda Butler. YouTube. https://www.youtube.com/watch?v=GVU_zANtroE&feature=youtu.be
A body system including a series of hollow organs joined in a long, twisting tube from the mouth to the anus. The hollow organs that make up the GI tract are the mouth, esophagus, stomach, small intestine, large intestine, and anus. The liver, pancreas, and gallbladder are the solid organs of the digestive system.
A hormone is a signaling molecule produced by glands in multicellular organisms that target distant organs to regulate physiology and behavior.
The body system which acts as a chemical messenger system comprising feedback loops of the hormones released by internal glands of an organism directly into the circulatory system, regulating distant target organs. In humans, the major endocrine glands are the thyroid gland and the adrenal glands.
The ability of an organism to maintain constant internal conditions despite external changes.
Created by CK-12 Foundation/Adapted by Christine Miller

Case Study Conclusion: Needing to Relax
As you learned in the beginning of this chapter, botulinum toxin — one form of which is sold under the brand name Botox — does much more than smooth out wrinkles. It can be used to treat a number of disorders involving excessive muscle contraction, including cervical dystonia. You also learned that cervical dystonia, which Edward suffers from, causes abnormal, involuntary muscle contractions of the neck. This results in jerky movements of the head and neck, and/or a sustained abnormal tilt to the head. It is often painful and can significantly interfere with a person’s life.

How could a toxin actually help treat a muscular disorder? The botulinum toxin is produced by the soil bacterium, Clostridium botulinum, and it is the cause of the potentially deadly disease called botulism. Botulism is often a foodborne illness, commonly caused by foods that are improperly canned. Other forms of botulism are caused by wound infections, or occur when infants consume spores of the bacteria from soil or honey.
Botulism can be life-threatening, because it paralyzes muscles throughout the body, including those involved in breathing. When a very small amount of botulinum toxin is injected carefully into specific muscles by a trained medical professional, however, it can be useful in inhibiting unwanted muscle contractions.
For cosmetic purposes, botulinum toxin injected into the facial muscles relaxes them to reduce the appearance of wrinkles. When used to treat cervical dystonia, it is injected into the muscles of the neck to inhibit excessive muscle contractions. For many patients, this helps relieve the abnormal positioning, movements, and pain associated with the disorder. The effect is temporary, so the injections must be repeated every three to four months to keep the symptoms under control.
How does botulinum toxin inhibit muscle contraction? First, recall how skeletal muscle contraction works. A motor neuron instructs skeletal muscle fibres to contract at a synapse between them called the neuromuscular junction. A nerve impulse called an action potential travels down to the axon terminal of the motor neuron, where it causes the release of the neurotransmitter acetylcholine (ACh) from synaptic vesicles. The ACh travels across the synaptic cleft and binds to ACh receptors on the muscle fibre, signaling the muscle fibre to contract. According to the sliding filament theory, the contraction of the muscle fibre occurs due to the sliding of myosin and actin filaments across each other. This causes the Z discs of the sacromeres to move closer together, shortening the sacromeres and causing the muscle fibre to contract.
If you wanted to inhibit muscle contraction, at what points could you theoretically interfere with this process? Inhibiting the action potential in the motor neuron, the release of ACh, the activity of ACh receptors, or the sliding filament process in the muscle fibre would all theoretically impair this process and inhibit muscle contraction. For example, in the disease myasthenia gravis, the function of the ACh receptors is impaired, causing a lack of sufficient muscle contraction. As you have learned, this results in muscle weakness that can eventually become life-threatening. Botulinum toxin works by inhibiting the release of ACh from the motor neurons, thereby removing the signal instructing the muscles to contract.
Fortunately, Edward’s excessive muscle contractions and associated pain improved significantly thanks to botulinum toxin injections. Although cervical dystonia cannot currently be cured, botulinum toxin injections have improved the quality of life for many patients with this and other disorders involving excessive involuntary muscle contractions.
As you have learned in this chapter, our muscular system allows us to do things like make voluntary movements, digest our food, and pump blood through our bodies. Whether they are in your arm, heart, stomach, or blood vessels, muscle tissue works by contracting. But as you have seen here, too much contraction can be a very bad thing. Fortunately, scientists and physicians have found a way to put a potentially deadly toxin — and wrinkle-reducing treatment — to excellent use as a medical treatment for some muscular system disorders.
Chapter 12 Summary
In this chapter, you learned about the muscular system. Specifically, you learned that:
- The muscular system consists of all the muscles of the body. There are three types of muscle: skeletal muscle (which is attached to bones by tendons and enables voluntary body movements), cardiac muscle (which makes up the walls of the heart and makes it beat) and smooth muscle (which is found in the walls of internal organs and other internal structures and controls their movements).
- Muscles are organs composed mainly of muscle cells, which may also be called muscle fibres or myocytes. Muscle cells are specialized for the function of contracting, which occurs when protein filaments inside the cells slide over one another using energy from ATP. Muscle tissue is the only type of tissue that has cells with the ability to contract.
- Muscles can grow larger, or hypertrophy. This generally occurs through increased use, although hormonal or other influences can also play a role. Muscles can also grow smaller, or atrophy. This may occur through lack of use, starvation, certain diseases, or aging. In both hypertrophy and atrophy, the size — but not the number — of muscle fibres changes. The size of muscles is the main determinant of muscle strength.
- Skeletal muscles need the stimulus of motor neurons to contract, and to move the body, they need the skeletal system to act upon.
- Skeletal muscle is the most common type of muscle tissue in the human body. To move bones in opposite directions, skeletal muscles often consist of pairs of muscles that work in opposition to one another to move bones in different directions at joints.
- Skeletal muscle fibres are bundled together in units called muscle fascicles, which are bundled together to form individual skeletal muscles. Skeletal muscles also have connective tissue supporting and protecting the muscle tissue.
-
- Each skeletal muscle fibre consists of a bundle of myofibrils, which are bundles of protein filaments. The filaments are arranged in repeating units called sarcomeres, which are the basic functional units of skeletal muscles. Skeletal muscle tissue is striated, because of the pattern of sarcomeres in its fibres.
- Skeletal muscle fibres can be divided into two types, called slow-twitch and fast-twitch fibres. Slow-twitch fibres are used mainly in aerobic endurance activities (such as long-distance running). Fast-twitch fibres are used mainly for non-aerobic, strenuous activities (such as sprinting). Proportions of the two types of fibres vary from muscle to muscle and person to person.
- Smooth muscle tissue is found in the walls of internal organs and vessels. When smooth muscles contract, they help the organs and vessels carry out their functions. The pattern of smooth muscle contraction to move substances through body tubes is called peristalsis. Contractions of smooth muscles are involuntary and controlled by the autonomic nervous system, hormones, and other substances.
-
- Cells of smooth muscle tissue are not striated because they lack sarcomeres, but the cells contract in the same basic way as striated muscle cells. Unlike striated muscle, smooth muscle can sustain very long-term contractions and maintain its contractile function, even when stretched.
- Cardiac muscle tissue is found only in the wall of the heart. When cardiac muscle contracts, the heart beats and pumps blood. Contractions of cardiac muscle are involuntary, like those of smooth muscles. They are controlled by electrical impulses from specialized cardiac cells.
-
- Like skeletal muscle, cardiac muscle is striated because its filaments are arranged in sarcomeres. The exact arrangement, however, differs, making cardiac and skeletal muscle tissues look different from one another.
- The heart is the muscle that performs the greatest amount of physical work in the course of a lifetime. Its cells contain a great many mitochondria to produce ATP for energy and to help the heart resist fatigue.
- A muscle contraction is an increase in the tension or a decrease in the length of a muscle. A muscle contraction is isometric if muscle tension changes, but muscle length remains the same. It is isotonic if muscle length changes, but muscle tension remains the same.
-
- A skeletal muscle contraction begins with electrochemical stimulation of a muscle fibre by a motor neuron. This occurs at a chemical synapse called a neuromuscular junction. The neurotransmitter acetylcholine diffuses across the synaptic cleft and binds to receptors on the muscle fibre. This initiates a muscle contraction.
- Once stimulated, the protein filaments within the skeletal muscle fibre slide past each other to produce a contraction. The sliding filament theory is the most widely accepted explanation for how this occurs. According to this theory, thick myosin filaments repeatedly attach to and pull on thin actin filaments, thus shortening sarcomeres.
- Crossbridge cycling is a cycle of molecular events that underlies the sliding filament theory. Using energy in ATP, myosin heads repeatedly bind with and pull on actin filaments. This moves the actin filaments toward the center of a sarcomere, shortening the sarcomere and causing a muscle contraction.
- The ATP needed for a muscle contraction comes first from ATP already available in the cell, and more is generated from creatine phosphate. These sources are quickly used up. Glucose and glycogen can be broken down to form ATP and pyruvate. Pyruvate can then be used to produce ATP in aerobic respiration if oxygen is available, or it can be used in anaerobic respiration if oxygen is not available.
- Physical exercise is defined as any bodily activity that enhances or maintains physical fitness and overall health. Activities such as household chores may even count as physical exercise! Current recommendations for adults are 30 minutes of moderate exercise a day.
- Aerobic exercise is any physical activity that uses muscles at less than their maximum contraction strength, but for long periods of time. This type of exercise uses a relatively high percentage of slow-twitch muscle fibres that consume large amounts of oxygen. Aerobic exercises increase cardiovascular endurance, and include cycling and brisk walking.
- Anaerobic exercise is any physical activity that uses muscles at close to their maximum contraction strength, but for short periods of time. This type of exercise uses a relatively high percentage of fast-twitch muscle fibres that consume small amounts of oxygen. Anaerobic exercises increase muscle and bone mass and strength, and they include push-ups and sprinting.
- Flexibility exercise is any physical activity that stretches and lengthens muscles, thereby improving range of motion and reducing risk of injury. Examples include stretching and yoga.
- Many studies have shown that physical exercise is positively correlated with a diversity of physical, mental, and emotional health benefits. Physical exercise also increases quality of life and life expectancy.
-
- Many of the benefits of exercise may come about because contracting muscles release hormones called myokines, which promote tissue repair and growth and have anti-inflammatory effects.
- Physical exercise can reduce risk factors for cardiovascular disease, including hypertension and excess body weight. Physical exercise can also increase factors associated with cardiovascular health, such as mechanical efficiency of the heart.
- Physical exercise has been shown to offer protection from dementia and other cognitive problems, perhaps because it increases blood flow or neurotransmitters in the brain, among other potential effects.
- Numerous studies suggest that regular aerobic exercise works as well as pharmaceutical antidepressants in treating mild-to-moderate depression, possibly because it increases synthesis of natural euphoriants in the brain.
- Research shows that physical exercise generally improves sleep for most people, and helps sleep disorders, such as insomnia. Other health benefits of physical exercise include better immune system function and reduced risk of type 2 diabetes and obesity.
- There is great variation in individual responses to exercise, partly due to genetic differences in proportions of slow-twitch and fast-twitch muscle fibres. People with more slow-twitch fibres may be able to develop greater endurance from aerobic exercise, whereas people with more fast-twitch fibres may be able to develop greater muscle size and strength from anaerobic exercise.
- Some adverse effects may occur if exercise is extremely intense and the body is not given proper rest between exercise sessions. Many people who overwork their muscles develop delayed onset muscle soreness (DOMS), which may be caused by tiny tears in muscle fibres.
- Musculoskeletal disorders are injuries that occur in muscles or associated tissues (such as tendons) because of biomechanical stresses. The disorders may be caused by sudden exertion, over-exertion, repetitive motions, and similar stresses.
-
- A muscle strain is an injury in which muscle fibres tear as a result of overstretching. First aid for a muscle strain includes the five steps represented by the acronym PRICE (protection, rest, ice, compression, and elevation). Medications for inflammation and pain (such as NSAIDs) may also be used.
- Tendinitis is inflammation of a tendon that occurs when it is over-extended or worked too hard without rest. Tendinitis may also be treated with PRICE and NSAIDs.
- Carpal tunnel syndrome is a biomechanical problem that occurs in the wrist when the median nerve becomes compressed between carpal bones. It may occur with repetitive use, a tumor, or trauma to the wrist. It may cause pain, numbness, and eventually — if untreated — muscle wasting in the thumb and first two fingers of the hand.
- Neuromuscular disorders are systemic disorders that occur because of problems with the nervous control of muscle contractions, or with the muscle cells themselves.
-
- Muscular dystrophy is a genetic disorder caused by defective proteins in muscle cells. It is characterized by progressive skeletal muscle weakness and death of muscle tissues.
- Myasthenia gravis is a genetic neuromuscular disorder characterized by fluctuating muscle weakness and fatigue. More muscles are affected, and muscles become increasingly weakened as the disorder progresses. Myasthenia gravis most often occurs because immune system antibodies block acetylcholine receptors on muscle cells, and because of the actual loss of acetylcholine receptors.
- Parkinson’s disease is a degenerative disorder of the central nervous system that mainly affects the muscular system and movement. It occurs because of the death of neurons in the midbrain. Characteristic signs of the disorder are muscle tremor, muscle rigidity, slowness of movement, and postural instability. Dementia and depression also often characterize advanced stages of the disease.
As you saw in this chapter, muscles need oxygen to provide enough ATP for most of their activities. In fact, all of the body’s systems require oxygen, and also need to remove waste products, such as carbon dioxide. In the next chapter, you will learn about how the respiratory system obtains and distributes oxygen throughout the body, as well as how it removes wastes, such as carbon dioxide.
Chapter 12 Review
-
- What are tendons? Name a muscular system disorder involving tendons
- Describe the relationship between muscles, muscle fibres, and fascicles.
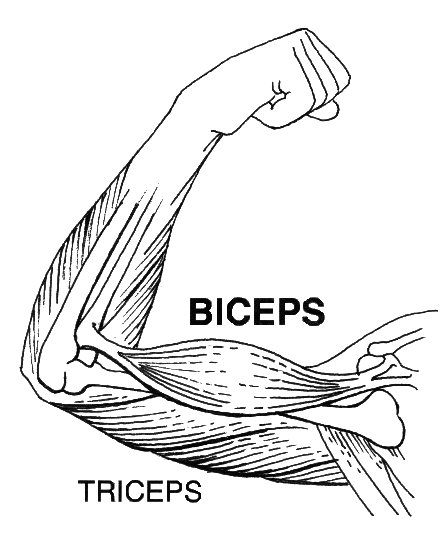
- The biceps and triceps muscles are shown above. Answer the following questions about these arm muscles.
- When the biceps contract and become shorter (as in the picture above), what kind of motion does this produce in the arm?
- Is the situation described in part (a) more likely to be an isometric or isotonic contraction? Explain your answer.
- If the triceps were to then contract, which way would the arm move?
- What are Z discs? What happens to them during muscle contraction?
- What is the function of mitochondria in muscle cells? Which type of muscle fibre has more mitochondria — slow-twitch or fast-twitch?
- What is the difference between primary and secondary Parkinson’s disease?
- Why can carpal tunnel syndrome cause muscle weakness in the hands?
Attributions
Figure 12.7.1
Botox, he whispered by Michael Reuter on Flickr is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/) license.
Figure 12.7.2
botulism by jason wilson on Flickr is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/) license.
Reference
Pearson Scott Foresman. (2020, April 14). File:Biceps (PSF).jpg [digital image]. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Biceps_(PSF).jpg&oldid=411251538. [Public Domain (https://en.wikipedia.org/wiki/Public_domain)]
Involuntary, striated muscle found only in the walls of the heart; also called myocardium.
A hollow, tube-like structure through which blood flows in the cardiovascular system; vein, artery, or capillary.

Created by: CK-12/Adapted by Christine Miller
Assembly Line
We stay alive because millions of different chemical reactions are taking place inside our bodies all the time. Each of our cells is like the busy auto assembly line pictured in Figure 3.10.1. Raw materials, half-finished products, and waste materials are constantly being used, produced, transported, and excreted. The "workers" on the cellular assembly line are mainly enzymes. These are the proteins that make biochemical reactions happen.
What Are Biochemical Reactions?
Chemical reactions that take place inside living things are called biochemical reactions. The sum of all the biochemical reactions in an organism is called metabolism. Metabolism includes both exothermic (energy-releasing) chemical reactions and endothermic (energy-absorbing) chemical reactions.
Catabolic Reactions
Exothermic reactions in organisms are called catabolic reactions. These reactions break down molecules into smaller units and release energy. An example of a catabolic reaction is the breakdown of glucose during cellular respiration, which releases energy that cells need to carry out life processes.
Anabolic Reactions
Endothermic reactions in organisms are called anabolic reactions. These reactions build up bigger molecules from smaller ones and absorb energy. An example of an anabolic reaction is the joining of amino acids to form a protein. Which type of reactions — catabolic or anabolic — do you think occur when your body digests food?
Enzymes
Most of the biochemical reactions that happen inside of living organisms require help. Why is this the case? For one thing, temperatures inside living things are usually too low for biochemical reactions to occur quickly enough to maintain life. The concentrations of reactants may also be too low for them to come together and react. Where do the biochemical reactions get the help they need to proceed? From the enzymes.
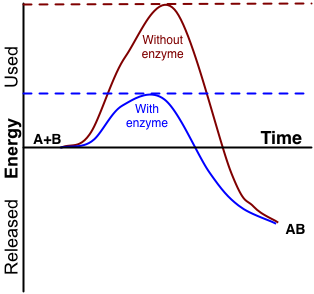
An enzyme is a protein that speeds up a biochemical reaction. It is a biological catalyst. An enzyme generally works by reducing the amount of activation energy needed to start the reaction. The graph in Figure 3.10.2 shows the activation energy needed for glucose to combine with oxygen. Less activation energy is needed when the correct enzyme is present than when it is not present.
An enzyme speeds up the reaction by lowering the required activation energy. Compare the activation energy needed with and without the enzyme.
How Well Enzymes Work
Enzymes are involved in most biochemical reactions, and they do their jobs extremely well. A typical biochemical reaction that would take several days or even several centuries to happen without an enzyme is likely to occur in just a split second with the proper enzyme! Without enzymes to speed up biochemical reactions, most organisms could not survive.
Enzymes are substrate-specific. The substrate of an enzyme is the specific substance it affects. Each enzyme works only with a particular substrate, which explains why there are so many different enzymes. In addition, for an enzyme to work, it requires specific conditions, such as the right temperature and pH. Some enzymes work best under acidic conditions, for example, while others work best in neutral environments.
Enzyme-Deficiency Disorders
There are hundreds of known inherited metabolic disorders in humans. In most of them, a single enzyme is either not produced by the body at all, or is otherwise produced in a form that doesn't work. The missing or defective enzyme is like an absentee worker on the cell's assembly line. Imagine the auto assembly line from the image at the start of this section. What if the worker who installed the steering wheel was absent? How would this impact the overall functioning of the vehicle? When an enzyme is missing, toxic chemicals build up, or an essential product isn't made. Generally, the normal enzyme is missing because the individual with the disorder inherited two copies of a gene mutation, which may have originated many generations previously.
Any given inherited metabolic disorder is generally quite rare in the general population. However, there are so many different metabolic disorders that a total of one in 1,000 to 2,500 newborns can be expected to have one.
3.10 Summary
- Biochemical reactions are chemical reactions that take place inside of living things. The sum of all of the biochemical reactions in an organism is called metabolism.
- Metabolism includes catabolic reactions, which are energy-releasing (exothermic) reactions, as well as anabolic reactions, which are energy-absorbing (endothermic) reactions.
- Most biochemical reactions need a biological catalyst called an enzyme to speed up the reaction. Enzymes reduce the amount of activation energy needed for the reaction to begin. Most enzymes are proteins that affect just one specific substance, which is called the enzyme's substrate.
- There are many inherited metabolic disorders in humans. Most of them are caused by a single defective or missing enzyme.
3.10 Review Questions
- What are biochemical reactions?
- Define metabolism.
- Compare and contrast catabolic and anabolic reactions.
- Explain the role of enzymes in biochemical reactions.
- What are enzyme-deficiency disorders?
- Explain why the relatively low temperature of living things, along with the low concentration of reactants, would cause biochemical reactions to occur very slowly in the body without enzymes.
- Answer the following questions about what happens after you eat a sandwich.
- Pieces of the sandwich go into your stomach, where there are digestive enzymes that break down the food. Which type of metabolic reaction is this? Explain your answer.
- During the process of digestion, some of the sandwich is broken down into glucose, which is then further broken down to release energy that your cells can use. Is this an exothermic endothermic reaction? Explain your answer.
- The proteins in the cheese, meat, and bread in the sandwich are broken down into their component amino acids. Then your body uses those amino acids to build new proteins. Which kind of metabolic reaction is represented by the building of these new proteins? Explain your answer.
- Explain why your body doesn’t just use one or two enzymes for all of its biochemical reactions.
- A ________ is the specific substance that an enzyme affects in a biochemical reaction.
- An enzyme is a biological _____________ .
- catabolism
- form of activation energy
- catalyst
- reactant
3.10 Explore More
https://www.youtube.com/watch?v=qgVFkRn8f10&feature=youtu.be
Enzymes (Updated), by The Amoeba Sisters, 2016.
https://www.youtube.com/watch?v=8m6RtOpqvtU&feature=youtu.be
What triggers a chemical reaction? - Kareem Jarrah, TED-Ed, 2015.
Figure 3.10.1
Auto Assembly line by Brian Snelson on Wikimedia Commons is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0) license.
Figure 3.10.2
Enzyme_activation_energy by G. Andruk [IMeowbot at the English language Wikipedia], is used under a CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0/) license.
References
Amoeba Sisters. (2016, August 28). Enzymes (updated). YouTube. https://www.youtube.com/watch?v=qgVFkRn8f10&feature=youtu.be
TED-Ed. (2015, January 15). What triggers a chemical reaction? - Kareem Jarrah. YouTube. https://www.youtube.com/watch?v=8m6RtOpqvtU&feature=youtu.be
The smallest type of blood vessel that connects arterioles and venules and that transfers substances between blood and tissues.
Created by: CK-12/Adapted by Christine Miller

The Blue Marble
It's often called the "water planet," and it's been given the nickname "the blue marble." You probably just call it "home." Almost three-quarters of our home planet is covered by water, and without it, life as we know it could not exist on Earth. Water, like carbon, has a special role in living things: it is needed by all known forms of life. Although water consists of simple molecules, each containing just three atoms, its structure gives it unique properties that help explain why it is vital to all living organisms.
Water, Water Everywhere
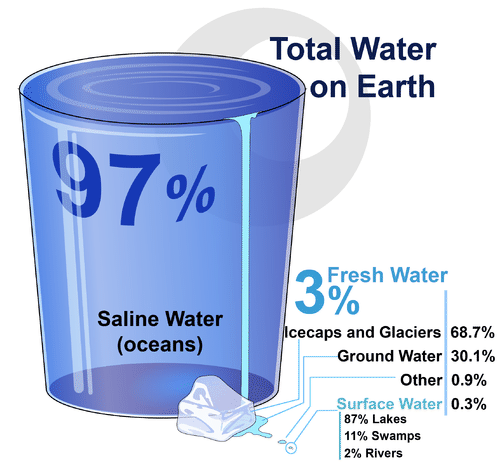
If you look at Figure 3.11.2, you will see where Earth’s water is found. The term water generally refers to its liquid state, and water is a liquid over a wide range of temperatures on Earth. Water, however, also occurs on Earth as a solid (ice) and as a gas (water vapor).
Structure and Properties of Water
You are likely already aware of some of the properties of water. For example, you know that water is tasteless and odorless. You also probably know that water is transparent, which means that light can pass through it. This is important for organisms that live in the water, because some of them need sunlight to make food by photosynthesis.
Chemical Structure of Water
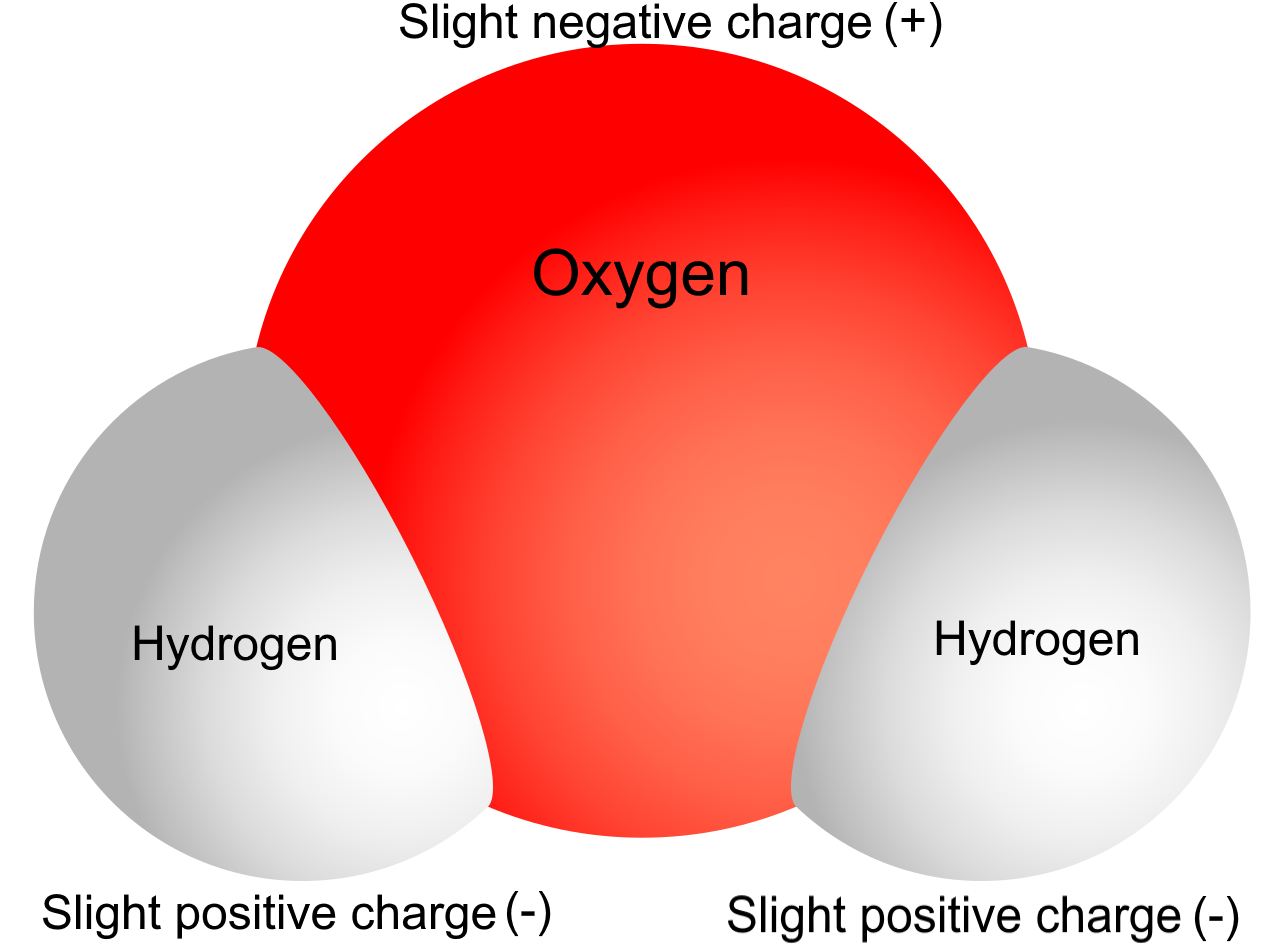
To understand some of water’s properties, you need to know more about its chemical structure. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen. The oxygen atom in a water molecule attracts electrons more strongly than the hydrogen atoms do. As a result, the oxygen atom has a slightly negative charge, and the hydrogen atoms have a slightly positive charge. A difference in electrical charge between different parts of the same molecule is called polarity. The diagram in Figure 3.11.3 shows water’s polarity.
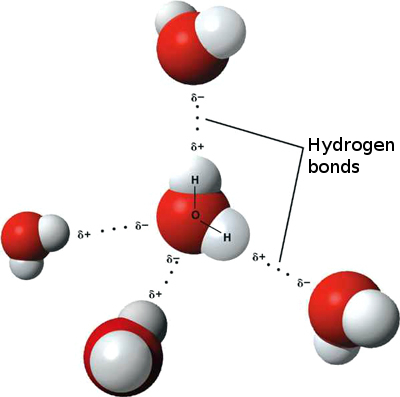
When it comes to charged molecules, opposites attract. In the case of water, the positive (hydrogen) end of one water molecule is attracted to the negative (oxygen) end of a nearby water molecule. Because of this attraction, weak bonds form between adjacent water molecules, as shown in Figure 3.11.4. The type of bond that forms between water molecules is called a hydrogen bond. Bonds between molecules are not as strong as bonds within molecules, but in water, they are strong enough to hold together nearby molecules.
How do you think hydrogen bonding affects water's properties?
Properties of Water

Hydrogen bonds between water molecules explain some of water’s properties — for example, why water molecules tend to "stick" together. Did you ever watch water drip from a leaky faucet or from a melting icicle? If you did, then you know that water always falls in drops, rather than as separate molecules. The dew drops pictured to the left are another example of water molecules sticking together.
Hydrogen bonds cause water to have a relatively high boiling point of 100°C (212°F). Extra energy is needed to break these bonds and separate water molecules so they can escape into the air as water vapor. Because of its high boiling point, most water on Earth is in a liquid state, rather than a gaseous state. Water in its liquid state is needed by all living things. Hydrogen bonds also cause water to expand when it freezes. This, in turn, causes ice to have a lower density (that is, less mass per unit volume) than liquid water. The lower density of ice means that it floats on water. In cold climates, ice floats on top of the water in lakes. This allows lake animals like fish to survive the winter by staying in the liquid water under the ice.
Watch the video below to hear more about hydrogen bonding and it's effects on the properties of water:
https://www.youtube.com/watch?v=UukRgqzk-KE
Why does ice float in water? - George Zaidan and Charles Morton, TED-ED, 2013.
Water and Living Things
The human body is about 70 per cent water (not counting the water in body fat, which varies from person to person). The body needs all this water to function normally. Just why is so much water required by human beings and other organisms? Water can dissolve many substances that organisms need. Water's polarity helps it dissolve other polar substances. Water is also necessary for many biochemical reactions. The examples below are among the most important biochemical processes that occur in living things, but they are just two of the many ways that water is involved in biochemical reactions.
- Photosynthesis: In this process, cells use the energy in sunlight to change carbon dioxide and water to glucose and oxygen. The reactions of photosynthesis can be represented by the chemical equation:
6CO2 + 6H2O + Energy → C6H12O6 + 6O2
- Cellular respiration: In this process, cells break down glucose in the presence of oxygen and release carbon dioxide, water, and energy. The reactions of cellular respiration can be represented by the chemical equation:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Water is involved in many other biochemical reactions and almost all life processes depend on water.
Feature: My Human Body

Are you a marathon runner or other endurance athlete? Do you live and work in a hot, humid climate? If you answered "yes" to either question, you may be at risk of water intoxication.
Water is considered the least toxic chemical compound, so it may surprise you to learn that drinking too much water can cause serious illness and even death. Water intoxication is a potentially fatal disturbance in brain functions. It results when the normal balance of sodium and other electrolytes in the body is pushed outside safe limits by overhydration, or taking in too much water. The condition is also called hyponatremia, which refers to a lower-than-normal level of sodium in the blood that occurs when more water is entering than leaving the body.
As excessive water is consumed, fluid outside the cells decreases in its concentration of sodium and other electrolytes relative to the concentration inside the cells. This causes fluid to enter the cells by osmosis to balance the electrolyte concentration. The extra fluid in the cells causes them to swell. In the brain, this swelling increases the pressure inside the skull. It is this increase in pressure that leads to the first observable symptoms of water intoxication, which typically include headache, confusion, irritability, and drowsiness. As the condition worsens, additional symptoms may occur, such as difficulty breathing during exertion, muscle weakness and pain, or nausea and vomiting. If the condition persists, the cells in the brain may swell to the point where blood flow is interrupted or pressure is applied to the brain stem. This is extremely dangerous and may lead to seizures, brain damage, coma, or even death.
Under normal circumstances, it is very rare to accidentally consume too much water. However, it is relatively common in athletes who participate in endurance activities, such as marathon running. A study conducted on participants of the 2002 Boston Marathon, for example, found that 13 per cent of the runners finished the race with water intoxication (Almond, et al., 2005). The study also found that water intoxication was just as likely to occur in runners who drank sports drinks containing electrolytes as those who drank plain water. Water intoxication is so common at marathon events that medical personnel who work at such events are trained to suspect water intoxication when runners collapse or show signs of confusion.
Because of the publicity water intoxication has received lately, sports experts have lowered their recommendations for water intake during endurance events. They now advise drinking only when thirsty rather than drinking to "stay ahead of thirst," which they recommended previously. Keeping water intake in line with water loss is the best way to prevent water intoxication. Mild water intoxication can be treated by restricting fluid intake. In more severe cases, treatment may require the use of diuretic drugs (which increase urination) or other types of drugs to reduce blood volume. Serious water intoxication should be considered a true medical emergency.
3.11 Summary
- Most water on Earth consists of salt water in the oceans. Only a tiny percentage of the Earth's water is fresh liquid water.
- Virtually all living things on Earth require liquid water. Water exists as a liquid over a wide range of temperatures and dissolves many substances. These properties depend on water's polarity, which causes water molecules to "stick" together.
- The human body is about 70 per cent water (outside of fat). Organisms need water to dissolve many substances and for most biochemical processes, including photosynthesis and cellular respiration.
3.11 Review Questions
- Where is most of Earth's fresh water found?
- Identify properties of water.
- What is polarity? Explain why water molecules are polar.
- Why do water molecules tend to "stick" together?
- What role does water play in photosynthesis and cellular respiration?
- Which do you think is stronger: the bonds between the hydrogen and oxygen atoms within a water molecule, or the bonds between the hydrogen and oxygen atoms between water molecules? Explain your answer.
- Given what you’ve learned about water intoxication (or hyponatremia), explain why you think drinking salt water would be bad for your cells.
- What is the name for the bonds that form between water molecules?
- Explain why water can dissolve other polar molecules.
- If there is pollution in the ocean that causes the water to become more cloudy or opaque, how do you think the ocean's photosynthetic organisms will be affected? Explain your answer.
- Describe one way in which your body gets rid of excess water.
- True or False: Ice floats on top of water because it is denser than water.
3.11 Explore More
https://www.youtube.com/watch?v=3jwAGWky98c&t=14s
Properties of Water, by The Amoeba Sisters, 2016.
https://www.youtube.com/watch?v=ASLUY2U1M-8&t=84s
How polarity makes water behave strangely - Christina Kleinberg, TED-Ed, 2013.
Attributions
Figure 3.11.1
Planet Earth by NASA (photo taken by either Harrison Schmitt or Ron Evans (of the Apollo 17 crew), on Wikimedia Commons, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 3.11.2
Total water on earth by LadyofHats at CK12, is used under a CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/) license.
Figure 3.11.3
Polarity of water by Christine Miller is released into the Public Domain (https://creativecommons.org/publicdomain/mark/1.0/).
Figure 3.11.4
Hydrogen bonds, translated by Michal Maňas (User:snek01) is released into the public domain (https://en.wikipedia.org/wiki/Public_domain). (Original uploader was Qwerter at Czech Wikipedia.)
Figure 3.11.5
Dew by Pascal Chanel on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 3.11.6
Woman in a wheelchair marathon by Kevin André on Unsplash is used under the Unsplash License (https://unsplash.com/license).
References
Almond, C.S., Shin, A.Y., Fortescue, E.B. et al. (2005, April). Hyponatremia among runners in the Boston Marathon. The New England Journal of Medicine, 352 (15), 1550–1624. doi:10.1056/NEJMoa043901. PMID 15829535.
Amoeba Sisters. (2016, July 26). Properties of Water. YouTube. https://www.youtube.com/watch?v=3jwAGWky98c&feature=youtu.be
Ruiz Villarreal, M. (LadyofHats). (2016, August 15). Figure 2. Total water on earth [digital image]. In Brainard, J., Henderson, R., CK-12's College Human Biology FlexBook® (section 3.11). CK12 Foundation. https://www.ck12.org/book/ck-12-college-human-biology/
TED-Ed. (2013, February 4). How polarity makes water behave strangely - Christina Kleinberg. YouTube. https://www.youtube.com/watch?v=ASLUY2U1M-8&feature=youtu.be
TED-Ed. (2013, October 22). Why does ice float in water? - George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=UukRgqzk-KE&feature=youtu.be
Structures containing neuronal cell bodies in the peripheral nervous system.
What Are You Made of?

Your entire body is made of cells and cells are made of molecules.If you look at your hand, what do you see? Of course, you see skin, which consists of cells. But what are skin cells made of? Like all living cells, they are made of matter. In fact, all things are made of matter. Matter is anything that takes up space and has mass. Matter, in turn, is made up of chemical substances. A chemical substance is matter that has a definite composition that is consistent throughout. A chemical substance may be either an element or a compound.
Elements and Atoms
An element is a pure substance. It cannot be broken down into other types of substances. Each element is made up of just one type of atom.
Structure of an Atom
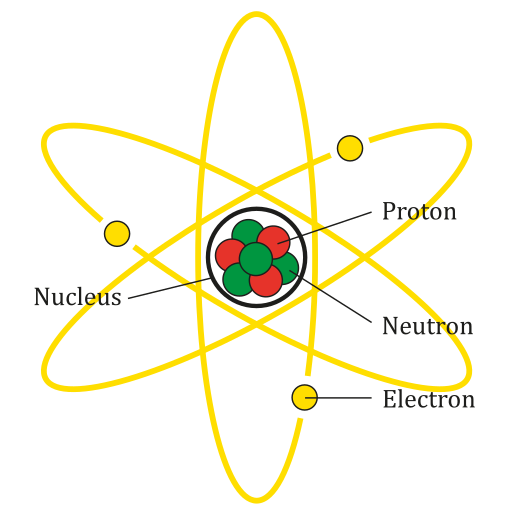
An atom is the smallest particle of an element that still has the properties of that element. Every substance is composed of atoms. Atoms are extremely small, typically about a ten-billionth of a metre in diametre. However, atoms do not have well-defined boundaries, as suggested by the atomic model shown below.
Every atom is composed of a central area — called the nucleus — and one or more subatomic particles called electrons, which move around the nucleus. The nucleus also consists of subatomic particles. It contains one or more protons and typically a similar number of neutrons. The number of protons in the nucleus determines the type of element an atom represents. An atom of hydrogen, for example, contains just one proton. Atoms of the same element may have different numbers of neutrons in the nucleus. Atoms of the same element with the same number of protons — but different numbers of neutrons — are called isotopes.
Protons have a positive electric charge and neutrons have no electric charge. Virtually all of an atom's mass is in the protons and neutrons in the nucleus. Electrons surrounding the nucleus have almost no mass, as well as a negative electric charge. If the number of protons and electrons in an atom are equal, then an atom is electrically neutral, because the positive and negative charges cancel each other out. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion.
The negatively-charged electrons of an atom are attracted to the positively-charged protons in the nucleus by a force called electromagnetic force, for which opposite charges attract. Electromagnetic force between protons in the nucleus causes these subatomic particles to repel each other, because they have the same charge. However, the protons and neutrons in the nucleus are attracted to each other by a different force, called nuclear force, which is usually stronger than the electromagnetic force. Nuclear force repels the positively-charged protons from each other.
Periodic Table of the Elements
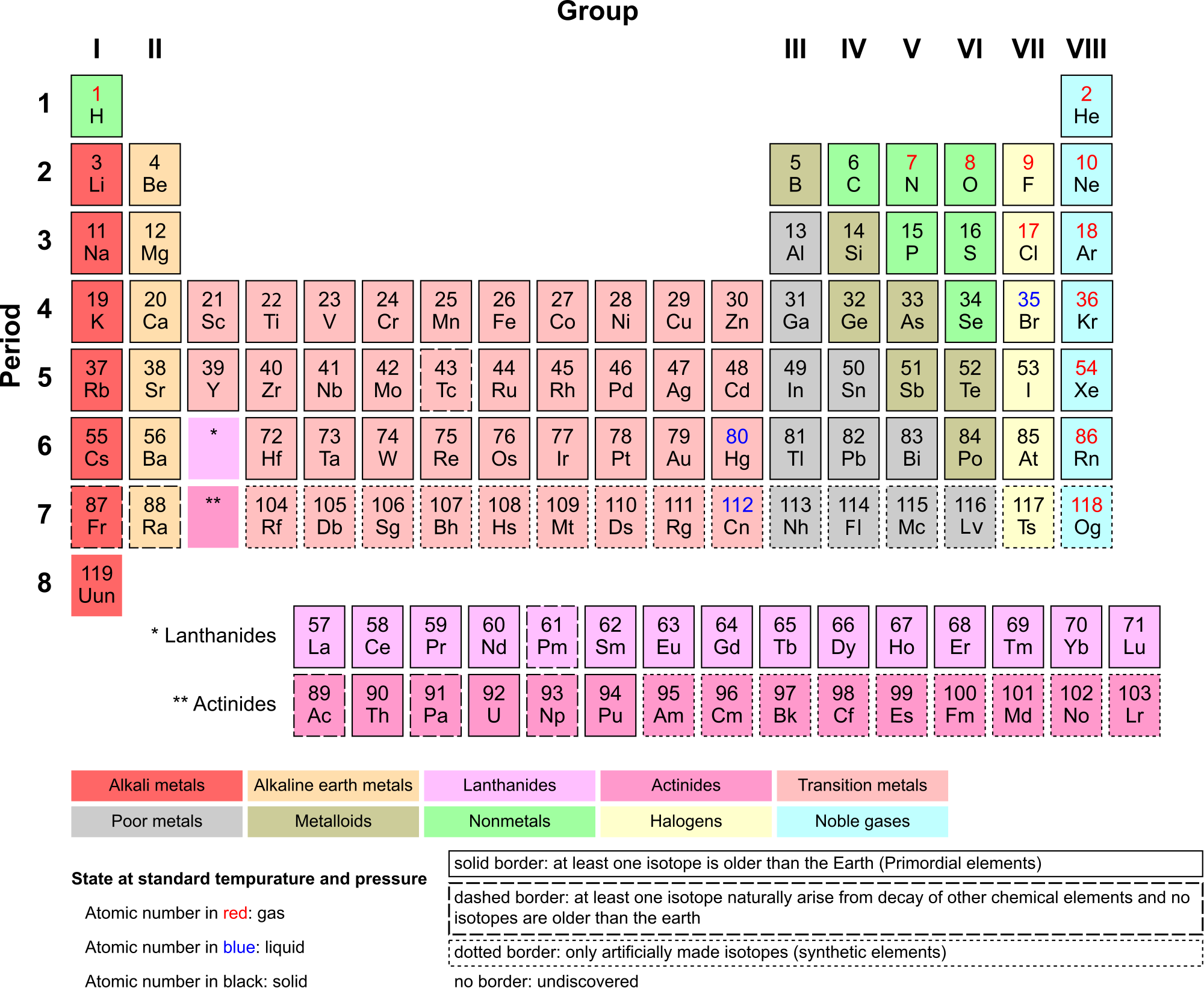
There are almost 120 known elements. As you can see in the Periodic Table of the Elements shown below, the majority of elements are metals. Examples of metals are iron (Fe) and copper (Cu). Metals are shiny and good conductors of electricity and heat. Nonmetal elements are far fewer in number. They include hydrogen (H) and oxygen (O). They lack the properties of metals.
The periodic table of the elements arranges elements in groups based on their properties. The element most important to life is carbon (C). Find carbon in the table. What type of element is it: metal or nonmetal?
Compounds and Molecules
A compound is a unique substance that consists of two or more elements combined in fixed proportions. This means that the composition of a compound is always the same. The smallest particle of most compounds in living things is called a molecule.
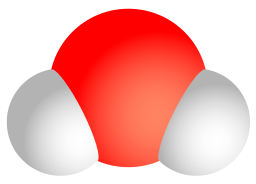
Consider water as an example. A molecule of water always contains one atom of oxygen and two atoms of hydrogen. The composition of water is expressed by the chemical formula H2O. A model of a water molecule is shown in Figure 3.2.4.
What causes the atoms of a water molecule to “stick” together? The answer is chemical bonds. A chemical bond is a force that holds together the atoms of molecules. Bonds in molecules involve the sharing of electrons among atoms. New chemical bonds form when substances react with one another. A chemical reaction is a process that changes some chemical substances into others. A chemical reaction is needed to form a compound, and another chemical reaction is needed to separate the substances in that compound.
3.2 Summary
- All matter consists of chemical substances. A chemical substance has a definite composition which is consistent throughout. A chemical substance may be either an element or a compound.
- An element is a pure substance that cannot be broken down into other types of substances.
- An atom is the smallest particle of an element that still has the properties of that element. Atoms, in turn, are composed of subatomic particles, including negative electrons, positive protons, and neutral neutrons. The number of protons in an atom determines the element it represents.
- Atoms have equal numbers of electrons and protons, so they have no charge. Ions are atoms that have lost or gained electrons, and as a result have either a positive or negative charge. Atoms with the same number of protons — but different numbers of neutrons — are called isotopes.
- There are almost 120 known elements. The majority of elements are metals. A smaller number are nonmetals. The latter include carbon, hydrogen, and oxygen.
- A compound is a substance that consists of two or more elements in a unique composition. The smallest particle of a compound is called a molecule. Chemical bonds hold together the atoms of molecules. Compounds can form only in chemical reactions, and they can break down only in other chemical reactions.
3.2 Review Questions
-
- What is an element? Give three examples.
- Define compound. Explain how compounds form.
- Compare and contrast atoms and molecules.
- The compound called water can be broken down into its constituent elements by applying an electric current to it. What ratio of elements is produced in this process?
- Relate ions and isotopes to elements and atoms.
- What is the most important element to life?
- Iron oxide is often known as rust — the reddish substance you might find on corroded metal. The chemical formula for this type of iron oxide is Fe2O3. Answer the following questions about iron oxide and briefly explain each answer.
- Is iron oxide an element or a compound?
- Would one particle of iron oxide be considered a molecule or an atom?
- Describe the relative proportion of atoms in iron oxide.
- What causes the Fe and O to stick together in iron oxide?
- Is iron oxide made of metal atoms, metalloid atoms, nonmetal atoms, or a combination of any of these?
- 14C is an isotope of carbon used in the radiocarbon dating of organic material. The most common isotope of carbon is 12C. Do you think 14C and 12C have different numbers of neutrons or protons? Explain your answer.
- Explain why ions have a positive or negative charge.
- Name the three subatomic particles described in this section.
3.2 Explore More
https://www.youtube.com/watch?v=yQP4UJhNn0I&feature=emb_logo
Just how small is an atom? TED-Ed, 2012
Attributions
Figure 3.2.1
Man Sitting, by Gregory Culmer, on Unsplash, is used under the Unsplash license (https://unsplash.com/license).
Figure 3.2.2
Lithium Atom diagram, by AG Caesar, is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0/deed.en)
Figure 3.2.3
Periodic Table Armtuk3, by Armtuk, is used under a CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/) license.
Figure 3.2.4
Water molecule, by Sakurambo, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
References
TED-Ed. (2012, April 16). Just how small is an atom. YouTube. https://www.youtube.com/watch?v=yQP4UJhNn0I&feature=youtu.be

Danger! Acid!
You probably know that batteries contain dangerous chemicals, including strong acids. Strong acids can hurt you if they come into contact with your skin or eyes. Therefore, it may surprise you to learn that your life depends on acids. There are many acids inside your body, and some of them are as strong as battery acid. Acids are needed for digestion and some forms of energy production. Genes are made of nucleic acids, proteins of amino acids, and lipids of fatty acids.
Water and Solutions
Acids (such as battery acid) are solutions. A solution is a mixture of two or more substances that has the same composition throughout. Many solutions are a mixture of water and some other substance. Not all solutions are acids. Some are bases and some are neither acids nor bases. To understand acids and bases, you need to know more about pure water.
In pure water (such as distilled water), a tiny fraction of water molecules naturally breaks down to form ions. An ion is an electrically charged atom or molecule. The breakdown of water is represented by the chemical equation:
2 H2O → H3O+ + OH-
The products of this reaction are a hydronium ion (H3O+) and a hydroxide ion (OH-). The hydroxide ion, which has a negative charge, forms when a water molecule gives up a positively charged hydrogen ion (H+). The hydronium ion, which has a positive charge, forms when another water molecule accepts the hydrogen ion.
Acidity and pH
The concentration of hydronium ions in a solution is known as acidity. In pure water, the concentration of hydronium ions is very low; only about one in ten million water molecules naturally breaks down to form a hydronium ion. As a result, pure water is essentially neutral. Acidity is measured on a scale called pH, as shown in Figure 3.12.2. Pure water has a pH of 7, so the point of neutrality on the pH scale is 7.
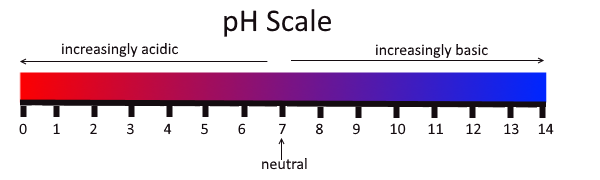
This pH scale shows the acidity of many common substances. The lower the pH value, the more acidic a substance is.

Acids
If a solution has a higher concentration of hydronium ions than pure water, it has a pH lower than 7. A solution with a pH lower than 7 is called an acid. As the hydronium ion concentration increases, the pH value decreases. Therefore, the more acidic a solution is, the lower its pH value is.
Did you ever taste vinegar? Like other acids, it tastes sour. Stronger acids can be harmful to organisms. Even stomach acid would eat through the stomach if it were not lined with a layer of mucus. Strong acids can also damage materials, even hard materials such as glass.
Bases
If a solution has a lower concentration of hydronium ions than pure water, it has a pH higher than 7. A solution with a pH higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. Like strong acids, strong bases can harm organisms and damage materials. For example, lye can burn the skin, and bleach can remove the colour from clothing.
Buffers
A buffer is a solution that can resist changes in pH. Buffers are able to maintain a certain pH by by absorbing any H+ or OH- ions added to the solution. Buffers are extremely important in biological systems in order to maintain a pH conducive to life. Bicarbonate is an example of a buffer which is used to maintain pH of the blood. In this buffering system, if blood becomes too acidic, carbonic acid will convert to carbon dioxide and water. If the blood becomes too basic, carbonic acid will convert to bicarbonate and H+ ions:
CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+
Acids, Bases, and Enzymes
Many acids and bases in living things provide the pH that enzymes need. Enzymes are biological catalysts that must work effectively for biochemical reactions to occur. Most enzymes can do their job only at a certain level of acidity. Cells secrete acids and bases to maintain the proper pH for enzymes to do their work.
Every time you digest food, acids and bases are at work in your digestive system. Consider the enzyme pepsin, which helps break down proteins in the stomach. Pepsin needs an acidic environment to do its job. The stomach secretes a strong acid called hydrochloric acid that allows pepsin to work. When stomach contents enter the small intestine, the acid must be neutralized, because enzymes in the small intestine need a basic environment in order to work. An organ called the pancreas secretes a base named bicarbonate into the small intestine, and this base neutralizes the acid.
Feature: My Human Body
Do you ever have heartburn? The answer is probably "yes." More than 60 million Americans have heartburn at least once a month, and more than 15 million suffer from it on a daily basis. Knowing more about heartburn may help you prevent it or know when it's time to seek medical treatment.
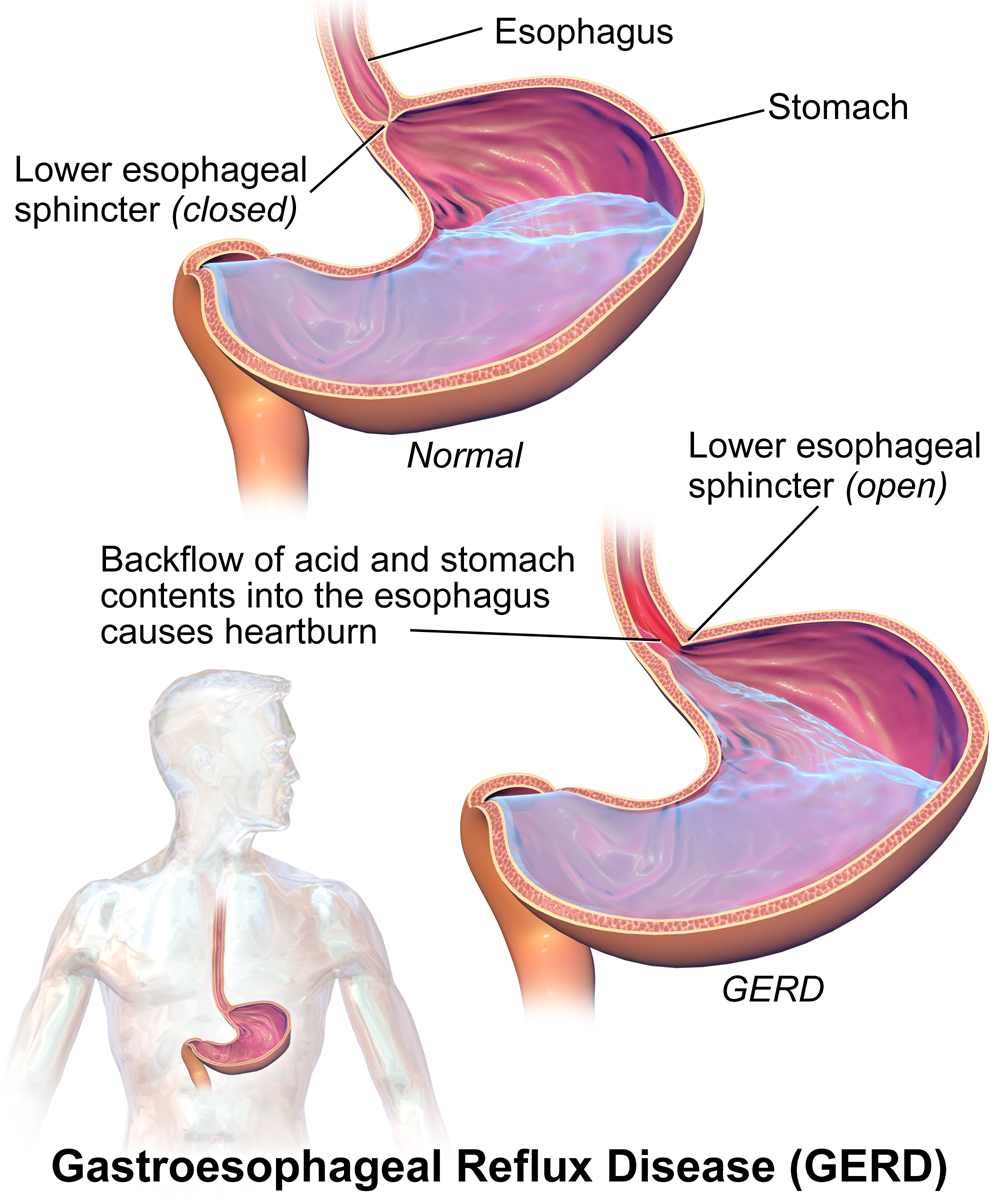
Heartburn doesn't have anything to do with the heart, but it does cause a burning sensation in the vicinity of the chest. Normally, the acid secreted into the stomach remains in the stomach where it is needed to allow pepsin to do its job of digesting proteins. A long tube called the esophagus carries food from the mouth to the stomach. A sphincter, or valve, between the esophagus and stomach opens to allow swallowed food to enter the stomach and then closes to prevent stomach contents from backflowing into the esophagus. If this sphincter is weak or relaxes inappropriately, stomach contents flow into the esophagus. Because stomach contents are usually acidic, this causes the burning sensation known as heartburn. People who are prone to heartburn and suffer from it often may be diagnosed with GERD, which stands for gastroesophageal reflux disease.
GERD — as well as occasional heartburn — often can be improved by dietary and other lifestyle changes that decrease the amount and acidity of reflux from the stomach into the esophagus.
- Some foods and beverages seem to contribute to GERD, so these should be avoided. Problematic foods include chocolate, fatty foods, peppermint, coffee, and alcoholic beverages.
- Decreasing portion size and eating the last meal of the day at least a couple of hours before bedtime may reduce the risk of reflux occurring.
- Smoking tends to weaken the lower esophageal sphincter, so quitting the habit may help control reflux.
- GERD is often associated with being overweight. Losing weight often brings improvement.
- Some people are helped by sleeping with the head of the bed elevated. This allows gravity to help control the backflow of acids into the esophagus from the stomach.
If you have frequent heartburn and lifestyle changes don't help, you may need medication to control the condition. Over-the-counter (OTC) antacids may be all that you need to control the occasional heartburn attack. OTC medications are usually bases that neutralize stomach acids. They may also create bubbles that help block stomach contents from entering the esophagus. For some people, OTC medications are not enough, and prescription medications are instead required for the control of GERD. These prescription medications generally work by inhibiting acid secretion in the stomach.
Be sure to see a doctor if you can't control your heartburn, or you have it often. Untreated GERD not only interferes with quality of life, it may also lead to more serious complications, ranging from esophageal bleeding to esophageal cancer.
3.12 Summary
- A solution is a mixture of two or more substances that has the same composition throughout. Many solutions consist of water and one or more dissolved substances.
- Acidity is a measure of the hydronium ion concentration in a solution. Pure water has a very low concentration and a pH of 7, which is the point of neutrality on the pH scale.
- Acids have a higher hydronium ion concentration than pure water and a pH lower than 7. Bases have a lower hydronium ion concentration than pure water and a pH higher than 7.
- Many acids and bases in living things are secreted to provide the proper pH for enzymes to work properly. Enzymes are the biological catalysts (like pepsin) needed to digest protein in the stomach. Pepsin requires an acidic environment.
3.12 Review Questions
-
- What is a solution?
- Define acidity.
- Explain how acidity is measured.
- Compare and contrast acids and bases.
- Hydrochloric acid is secreted by the stomach to provide an acidic environment for the enzyme pepsin. What is the pH of this acid? How strong of an acid is it compared with other acids?
- Define an ion. Identify the ions in the equation below, and explain what makes them ions:
- 2 H2O → H3O+ + OH-
- Explain why the pancreas secretes bicarbonate into the small intestine.
- Do you think pepsin would work in the small intestine? Why or why not?
- You may have mixed vinegar and baking soda and noticed that they bubble and react with each other. Explain why this happens. Explain also what happens to the pH of this solution after you mix the vinegar and baking soda.
- Pregnancy hormones can cause the lower esophageal sphincter to relax. What effect do you think this has on pregnant women? Explain your answer.
3.12 Explore More
https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
pH and Buffers by Bozeman Science, 2014.
https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton, TED-Ed, 2013.
Attributions
Figure 3.12.1
Leaky battery by Carbon Arc on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
Figure 3.12.2
PH_Scale by Christinelmiller on Wikimedia Commons is used under a © CC0 1.0 (https://creativecommons.org/publicdomain/zero/1.0/) public domain dedication license.
Figure 3.12.3
Ph scale with examples by OpenStax College, on Wikimedia Commons, is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 3.12.4
GERD by BruceBlaus on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
References
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 26.15 The pH Scale [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/26-4-acid-base-balance
Bozeman Science. (2014, February 22). pH and buffers. YouTube. https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
TED-Ed. (2013, October 24). The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
The way in which scientists and researchers use a systematic approach to answer questions about the world around us.
a colorless cell that circulates in the blood and body fluids and is involved in counteracting foreign substances and disease; a white (blood) cell. There are several types, all amoeboid cells with a nucleus, including lymphocytes, granulocytes, monocytes, and macrophages.

Created by: CK-12/Adapted by Christine Miller
Danger! Acid!
You probably know that batteries contain dangerous chemicals, including strong acids. Strong acids can hurt you if they come into contact with your skin or eyes. Therefore, it may surprise you to learn that your life depends on acids. There are many acids inside your body, and some of them are as strong as battery acid. Acids are needed for digestion and some forms of energy production. Genes are made of nucleic acids, proteins of amino acids, and lipids of fatty acids.
Water and Solutions
Acids (such as battery acid) are solutions. A solution is a mixture of two or more substances that has the same composition throughout. Many solutions are a mixture of water and some other substance. Not all solutions are acids. Some are bases and some are neither acids nor bases. To understand acids and bases, you need to know more about pure water.
In pure water (such as distilled water), a tiny fraction of water molecules naturally breaks down to form ions. An ion is an electrically charged atom or molecule. The breakdown of water is represented by the chemical equation:
2 H2O → H3O+ + OH-
The products of this reaction are a hydronium ion (H3O+) and a hydroxide ion (OH-). The hydroxide ion, which has a negative charge, forms when a water molecule gives up a positively charged hydrogen ion (H+). The hydronium ion, which has a positive charge, forms when another water molecule accepts the hydrogen ion.
Acidity and pH
The concentration of hydronium ions in a solution is known as acidity. In pure water, the concentration of hydronium ions is very low; only about one in ten million water molecules naturally breaks down to form a hydronium ion. As a result, pure water is essentially neutral. Acidity is measured on a scale called pH, as shown in Figure 3.12.2. Pure water has a pH of 7, so the point of neutrality on the pH scale is 7.

This pH scale shows the acidity of many common substances. The lower the pH value, the more acidic a substance is.

Acids
If a solution has a higher concentration of hydronium ions than pure water, it has a pH lower than 7. A solution with a pH lower than 7 is called an acid. As the hydronium ion concentration increases, the pH value decreases. Therefore, the more acidic a solution is, the lower its pH value is.
Did you ever taste vinegar? Like other acids, it tastes sour. Stronger acids can be harmful to organisms. Even stomach acid would eat through the stomach if it were not lined with a layer of mucus. Strong acids can also damage materials, even hard materials such as glass.
Bases
If a solution has a lower concentration of hydronium ions than pure water, it has a pH higher than 7. A solution with a pH higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. Like strong acids, strong bases can harm organisms and damage materials. For example, lye can burn the skin, and bleach can remove the colour from clothing.
Buffers
A buffer is a solution that can resist changes in pH. Buffers are able to maintain a certain pH by by absorbing any H+ or OH- ions added to the solution. Buffers are extremely important in biological systems in order to maintain a pH conducive to life. Bicarbonate is an example of a buffer which is used to maintain pH of the blood. In this buffering system, if blood becomes too acidic, carbonic acid will convert to carbon dioxide and water. If the blood becomes too basic, carbonic acid will convert to bicarbonate and H+ ions:
CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+
Acids, Bases, and Enzymes
Many acids and bases in living things provide the pH that enzymes need. Enzymes are biological catalysts that must work effectively for biochemical reactions to occur. Most enzymes can do their job only at a certain level of acidity. Cells secrete acids and bases to maintain the proper pH for enzymes to do their work.
Every time you digest food, acids and bases are at work in your digestive system. Consider the enzyme pepsin, which helps break down proteins in the stomach. Pepsin needs an acidic environment to do its job. The stomach secretes a strong acid called hydrochloric acid that allows pepsin to work. When stomach contents enter the small intestine, the acid must be neutralized, because enzymes in the small intestine need a basic environment in order to work. An organ called the pancreas secretes a base named bicarbonate into the small intestine, and this base neutralizes the acid.
Feature: My Human Body
Do you ever have heartburn? The answer is probably "yes." More than 60 million Americans have heartburn at least once a month, and more than 15 million suffer from it on a daily basis. Knowing more about heartburn may help you prevent it or know when it's time to seek medical treatment.

Heartburn doesn't have anything to do with the heart, but it does cause a burning sensation in the vicinity of the chest. Normally, the acid secreted into the stomach remains in the stomach where it is needed to allow pepsin to do its job of digesting proteins. A long tube called the esophagus carries food from the mouth to the stomach. A sphincter, or valve, between the esophagus and stomach opens to allow swallowed food to enter the stomach and then closes to prevent stomach contents from backflowing into the esophagus. If this sphincter is weak or relaxes inappropriately, stomach contents flow into the esophagus. Because stomach contents are usually acidic, this causes the burning sensation known as heartburn. People who are prone to heartburn and suffer from it often may be diagnosed with GERD, which stands for gastroesophageal reflux disease.
GERD — as well as occasional heartburn — often can be improved by dietary and other lifestyle changes that decrease the amount and acidity of reflux from the stomach into the esophagus.
- Some foods and beverages seem to contribute to GERD, so these should be avoided. Problematic foods include chocolate, fatty foods, peppermint, coffee, and alcoholic beverages.
- Decreasing portion size and eating the last meal of the day at least a couple of hours before bedtime may reduce the risk of reflux occurring.
- Smoking tends to weaken the lower esophageal sphincter, so quitting the habit may help control reflux.
- GERD is often associated with being overweight. Losing weight often brings improvement.
- Some people are helped by sleeping with the head of the bed elevated. This allows gravity to help control the backflow of acids into the esophagus from the stomach.
If you have frequent heartburn and lifestyle changes don't help, you may need medication to control the condition. Over-the-counter (OTC) antacids may be all that you need to control the occasional heartburn attack. OTC medications are usually bases that neutralize stomach acids. They may also create bubbles that help block stomach contents from entering the esophagus. For some people, OTC medications are not enough, and prescription medications are instead required for the control of GERD. These prescription medications generally work by inhibiting acid secretion in the stomach.
Be sure to see a doctor if you can't control your heartburn, or you have it often. Untreated GERD not only interferes with quality of life, it may also lead to more serious complications, ranging from esophageal bleeding to esophageal cancer.
3.12 Summary
- A solution is a mixture of two or more substances that has the same composition throughout. Many solutions consist of water and one or more dissolved substances.
- Acidity is a measure of the hydronium ion concentration in a solution. Pure water has a very low concentration and a pH of 7, which is the point of neutrality on the pH scale.
- Acids have a higher hydronium ion concentration than pure water and a pH lower than 7. Bases have a lower hydronium ion concentration than pure water and a pH higher than 7.
- Many acids and bases in living things are secreted to provide the proper pH for enzymes to work properly. Enzymes are the biological catalysts (like pepsin) needed to digest protein in the stomach. Pepsin requires an acidic environment.
3.12 Review Questions
-
- What is a solution?
- Define acidity.
- Explain how acidity is measured.
- Compare and contrast acids and bases.
- Hydrochloric acid is secreted by the stomach to provide an acidic environment for the enzyme pepsin. What is the pH of this acid? How strong of an acid is it compared with other acids?
- Define an ion. Identify the ions in the equation below, and explain what makes them ions:
- 2 H2O → H3O+ + OH-
- Explain why the pancreas secretes bicarbonate into the small intestine.
- Do you think pepsin would work in the small intestine? Why or why not?
- You may have mixed vinegar and baking soda and noticed that they bubble and react with each other. Explain why this happens. Explain also what happens to the pH of this solution after you mix the vinegar and baking soda.
- Pregnancy hormones can cause the lower esophageal sphincter to relax. What effect do you think this has on pregnant women? Explain your answer.
3.12 Explore More
https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
pH and Buffers by Bozeman Science, 2014.
https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton, TED-Ed, 2013.
Attributions
Figure 3.12.1
Leaky battery by Carbon Arc on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
Figure 3.12.2
PH_Scale by Christinelmiller on Wikimedia Commons is used under a © CC0 1.0 (https://creativecommons.org/publicdomain/zero/1.0/) public domain dedication license.
Figure 3.12.3
Ph scale with examples by OpenStax College, on Wikimedia Commons, is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 3.12.4
GERD by BruceBlaus on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
References
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 26.15 The pH Scale [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/26-4-acid-base-balance
Bozeman Science. (2014, February 22). pH and buffers. YouTube. https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
TED-Ed. (2013, October 24). The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
After reading this chapter, you should be able to see numerous connections between chemistry, human life, and health. In Joseph’s situation, chemistry is involved in the reasons why his father has diabetes, why his personal risk of getting diabetes is high, and why the dietary changes he is considering could be effective.
Type 2 diabetes affects populations worldwide and is caused primarily by a lack of response in the body to the hormone insulin, which causes problems in the regulation of blood sugar, or glucose. Insulin is a peptide hormone, and as you have learned, peptides are chains of amino acids. Therefore, insulin is in the class of biochemical compounds called proteins. Joseph is at increased risk of diabetes partly because there is a genetic component to the disease. DNA, which is a type of chemical compound called a nucleic acid, is passed down from parents to their offspring, and carries the instructions for the production of proteins in units called genes. If there is a problem in a gene (or genes) that contributes to the development of a disease, such as type 2 diabetes, this can get passed down to the offspring and may raise that child’s risk of getting the disease.
But genetics is only part of the reason why Joseph is at an increased risk of diabetes. Obesity itself is a risk factor, and one that can be shared in families due to shared lifestyle factors (such as poor diet and lack of exercise), as well as genetics. Consumption of too many refined carbohydrates (like white bread and soda) may also contribute to obesity and the development of diabetes. As you probably now know, these simple carbohydrates are more easily and quickly broken down in the digestive system into glucose than larger complex carbohydrate molecules, such as those found in vegetables and whole grains. This can lead to dramatic spikes in blood sugar levels, which is particularly problematic for people with diabetes because they have trouble maintaining their blood sugar at a safe level. You can understand why Joseph’s father limits his consumption of refined carbohydrates, and in fact, some scientific studies have shown that avoiding refined carbohydrates may actually help reduce the risk of getting diabetes in the first place.

Joseph’s friend recommended eating a low fat, high carbohydrate diet to lose weight, but you can see that the type of carbohydrate — simple or complex — is an important consideration. Eating a large amount of white bread and rice may not help Joseph reduce his risk of diabetes, but a healthy diet that helps him lose weight may lower his risk of diabetes, since obesity itself is a factor. Which specific diet will work best to help him lose weight probably depends on a variety of factors, including his biology, lifestyle, and food preferences. Joseph should consult with his doctor about his diet and exercise plan, so that his specific situation can be taken into account and monitored by a medical professional.
Drinking enough water is usually good advice for everyone, especially if it replaces sugary drinks like soda. You now know that water is important for many of the chemical reactions that take place in the body. But you can have too much of a good thing — as in the case of marathon runners who can make themselves sick from drinking too much water! As you can see, proper balance, or homeostasis, is very important to the health of living organisms.
Finally, you probably now realize that “chemicals” do not have to be scary, toxic substances. All matter consists of chemicals, including water, your body, and healthy fresh fruits and vegetables, like the ones pictured in Figure 3.12.2. When people advocate “clean eating” and avoiding “chemicals” in food, they are usually referring to avoiding synthetic — or man-made — chemical additives, such as preservatives. This can be a healthy way to eat because it involves eating a variety of whole, fresh, unprocessed foods. But there is no reason to be scared of chemicals in general — they are simply molecules and how they react depends on what they are, what other molecules are present, and the environmental conditions surrounding them.
Chapter 3 Summary
By now, you should have a good understanding of the basics of the chemistry of life. Specifically, you have learned:
- All matter consists of chemical substances. A chemical substance has a definite and consistent composition and may be either an element or a compound.
- An element is a pure substance that cannot be broken down into other types of substances.
- An atom is the smallest particle of an element that still has the properties of that element. Atoms, in turn, are composed of subatomic particles, including negative electrons, positive protons, and neutral neutrons. The number of protons in an atom determines the element it represents.
- Atoms have equal numbers of electrons and protons, so they have no charge. Ions are atoms that have lost or gained electrons, so they have either a positive or negative charge. Atoms with the same number of protons but different numbers of neutrons are called isotopes.
- There are almost 120 known elements. The majority of elements are metals. A smaller number are nonmetals, including carbon, hydrogen, and oxygen.
- A compound is a substance that consists of two or more elements in a unique composition. The smallest particle of a compound is called a molecule. Chemical bonds hold together the atoms of molecules. Compounds can form only in chemical reactions, and they can break down only in other chemical reactions.
- Biochemical compounds are carbon-based compounds found in living things. They make up cells and other structures of organisms and carry out life processes. Most biochemical compounds are large molecules called polymers that consist of many repeating units of smaller molecules called monomers.
- There are millions of different biochemical compounds, but all of them fall into four major classes: carbohydrates, lipids, proteins, and nucleic acids.
- Carbohydrates are the most common class of biochemical compounds. They provide cells with energy, store energy, and make up organic structures, such as the cell walls of plants. The basic building block of carbohydrates is the monosaccharide.
- Sugars are short-chain carbohydrates that supply us with energy. Simple sugars, such as glucose, consist of just one monosaccharide. Some sugars, such as sucrose (or table sugar) consist of two monosaccharides and are called disaccharides.
- Complex carbohydrates, or polysaccharides, consist of hundreds or even thousands of monosaccharides. They include starch, glycogen, cellulose, and chitin.
- Starch is made by plants to store energy and is readily broken down into its component sugars during digestion.
- Glycogen is made by animals and fungi to store energy and plays a critical part in the homeostasis of blood glucose levels in humans.
- Cellulose is the most common biochemical compound in living things. It forms the cell walls of plants and certain algae. Humans cannot digest cellulose, but it makes up most of the crucial dietary fibre in the human diet.
- Chitin makes up organic structures, such as the cell walls of fungi and the exoskeletons of insects and other arthropods.
- Lipids include fats and oils. They store energy, form cell membranes, and carry messages.
- Lipid molecules consist mainly of repeating units called fatty acids. Fatty acids may be saturated or unsaturated, depending on the proportion of hydrogen atoms they contain. Animals store fat as saturated fatty acids, while plants store fat as unsaturated fatty acids.
- Types of lipids include triglycerides, phospholipids, and steroids.
- Triglycerides contain glycerol (an alcohol) in addition to fatty acids. Humans and other animals store fat as triglycerides in fat cells.
- Phospholipids contain phosphate and glycerol in addition to fatty acids. They are the main component of cell membranes in all living things.
- Steroids are lipids with a four-ring structure. Some steroids, such as cholesterol, are important components of cell membranes. Many other steroids are hormones.
- In living things, proteins include enzymes, antibodies, and numerous other important compounds. They help cells keep their shape, make up muscles, speed up chemical reactions, and carry messages and materials (among other functions).
- Proteins are made up of small monomer molecules called amino acids.
- Long chains of amino acids form polypeptides. The sequence of amino acids in polypeptides makes up the primary structure of proteins. Secondary structure refers to configurations such as helices and sheets within polypeptide chains. Tertiary structure is a protein's overall three-dimensional shape, which controls the molecule's basic function. A quaternary structure forms if multiple protein molecules join together and function as a complex.
- The chief characteristic that allows proteins' diverse functions is their ability to bind specifically and tightly with other molecules.
- Nucleic acids include DNA and RNA. They encode instructions for making proteins, helping make proteins, and passing the encoded instructions from parents to offspring.
- Nucleic acids are built of monomers called nucleotides, which bind together in long chains to form polynucleotides. DNA consists of two polynucleotides, and RNA consists of one polynucleotide.
- Each nucleotide consists of a sugar molecule, phosphate group, and nitrogen base. Sugars and phosphate groups of adjacent nucleotides bind together to form the "backbone" of the polynucleotide. Bonds between complementary bases hold together the two polynucleotide chains of DNA and cause it to take on its characteristic double helix shape.
- DNA makes up genes, and the sequence of nitrogen bases in DNA makes up the genetic code for the synthesis of proteins. RNA helps synthesize proteins in cells. The genetic code in DNA is also passed from parents to offspring during reproduction, explaining how inherited characteristics are passed from one generation to the next.
- A chemical reaction is a process that changes some chemical substances into others. A substance that starts a chemical reaction is called a reactant, and a substance that forms in a chemical reaction is called a product. During the chemical reaction, bonds break in reactants and new bonds form in products.
- Chemical reactions can be represented by chemical equations. According to the law of conservation of mass, mass is always conserved in a chemical reaction, so a chemical equation must be balanced, with the same number of atoms of each type of element in the products as in the reactants.
- Many chemical reactions occur all around us each day, such as iron rusting and organic matter rotting, but not all changes are chemical processes. Some changes, such as ice melting or paper being torn into smaller pieces, are physical processes that do not involve chemical reactions and the formation of new substances.
- All chemical reactions involve energy, and they require activation energy to begin. Exothermic reactions release energy. Endothermic reactions absorb energy.
- Biochemical reactions are chemical reactions that take place inside living things. The sum of all the biochemical reactions in an organism is called metabolism. Metabolism includes catabolic reactions (exothermic reactions) and anabolic reactions (endothermic reactions).
- Most biochemical reactions require a biological catalyst called an enzyme to speed up the reaction by reducing the amount of activation energy needed for the reaction to begin. Most enzymes are proteins that affect just one specific substance, called the enzyme's substrate.
- Virtually all living things on Earth require liquid water. Only a tiny per cent of Earth's water is fresh liquid water. Water exists as a liquid over a wide range of temperatures, and it dissolves many substances. These properties depend on water's polarity, which causes water molecules to "stick" together through weak bonds called hydrogen bonds.
- The human body is about 70 per cent water (outside of fat). Organisms need water to dissolve many substances and for most biochemical processes, including photosynthesis and cellular respiration.
- A solution is a mixture of two or more substances that has the same composition throughout. Many solutions consist of water and one or more dissolved substances.
- Acidity is a measure of the hydronium ion concentration in a solution. Pure water has a very low concentration and a pH of 7, which is the point of neutrality on the pH scale. Acids have a higher hydronium ion concentration than pure water and a pH lower than 7. Bases have a lower hydronium ion concentration than pure water and a pH higher than 7.
- Many acids and bases in living things are secreted to provide the proper pH for enzymes to work properly.
Now you understand the chemistry of the molecules that make up living things. In the next chapter, you will learn how these molecules make up the basic unit of structure and function in living organisms — cells — and you will be able to understand some of the crucial chemical reactions that occur within cells.
Chapter 3 Review
-
- The chemical formula for the complex carbohydrate glycogen is C24H42O21.
- What are the elements in glycogen?
- How many atoms are in one molecule of glycogen?
- Is glycogen an ion? Why or why not?
- Is glycogen a monosaccharide or a polysaccharide? Besides memorizing this fact, how would you know this based on the information in the question?
- What is the function of glycogen in the human body?
- What is the difference between an ion and a polar molecule? Give an example of each in your explanation.
- Define monomer and polymer.
-
- What is the difference between a protein and a polypeptide?
-
- People with diabetes have trouble controlling the level of glucose in their bloodstream. Knowing this, why do you think it is often recommended that people with diabetes limit their consumption of carbohydrates?
- Identify each of the following reactions as endothermic or exothermic.
- cellular respiration
- photosynthesis
- catabolic reactions
- anabolic reactions
- Pepsin is an enzyme in the stomach that helps us digest protein. Answer the following questions about pepsin:
- What is the substrate for pepsin?
- How does pepsin work to speed up protein digestion?
- Given what you know about the structure of proteins, what do you think are some of the products of the reaction that pepsin catalyzes?
- The stomach is normally acidic. What do you think would happen to the activity of pepsin and protein digestion if the pH is raised significantly?
Attributions
Figure 3.13.1
Prevalence_of_Diabetes_by_Percent_of_Country_Population_(2014)_Gradient_Map by Walter Scott Wilkens [Wwilken2], University of Illinois - Urbana Champaign Department of Geography and GIScience, on Wikimedia Commons, is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
Figure 3.13.2
Healthy plate by Melinda Young Stuart on Flickr is used under a CC BY-NC-ND 2.0 (https://creativecommons.org/licenses/by-nc-nd/2.0/) license.


