13.6 Smoking and Health
Sure Death
This anti-smoking photo (Figure 13.6.1) clearly makes the point that smoking causes death. The image is not using hyperbole, because smoking actually is deadly. It causes about 7 million deaths each year, and is the single greatest cause of preventable death worldwide. As many as half of all people who smoke tobacco die from it. As a result of smoking’s deadly effects, the life expectancy of long-term smokers is significantly less than that of non-smokers. In fact, long-term smokers can expect their lifespan to be reduced by as much as 18 years, and they are three times more likely than non-smokers to die before the age of 70.
Why Is Smoking Deadly?
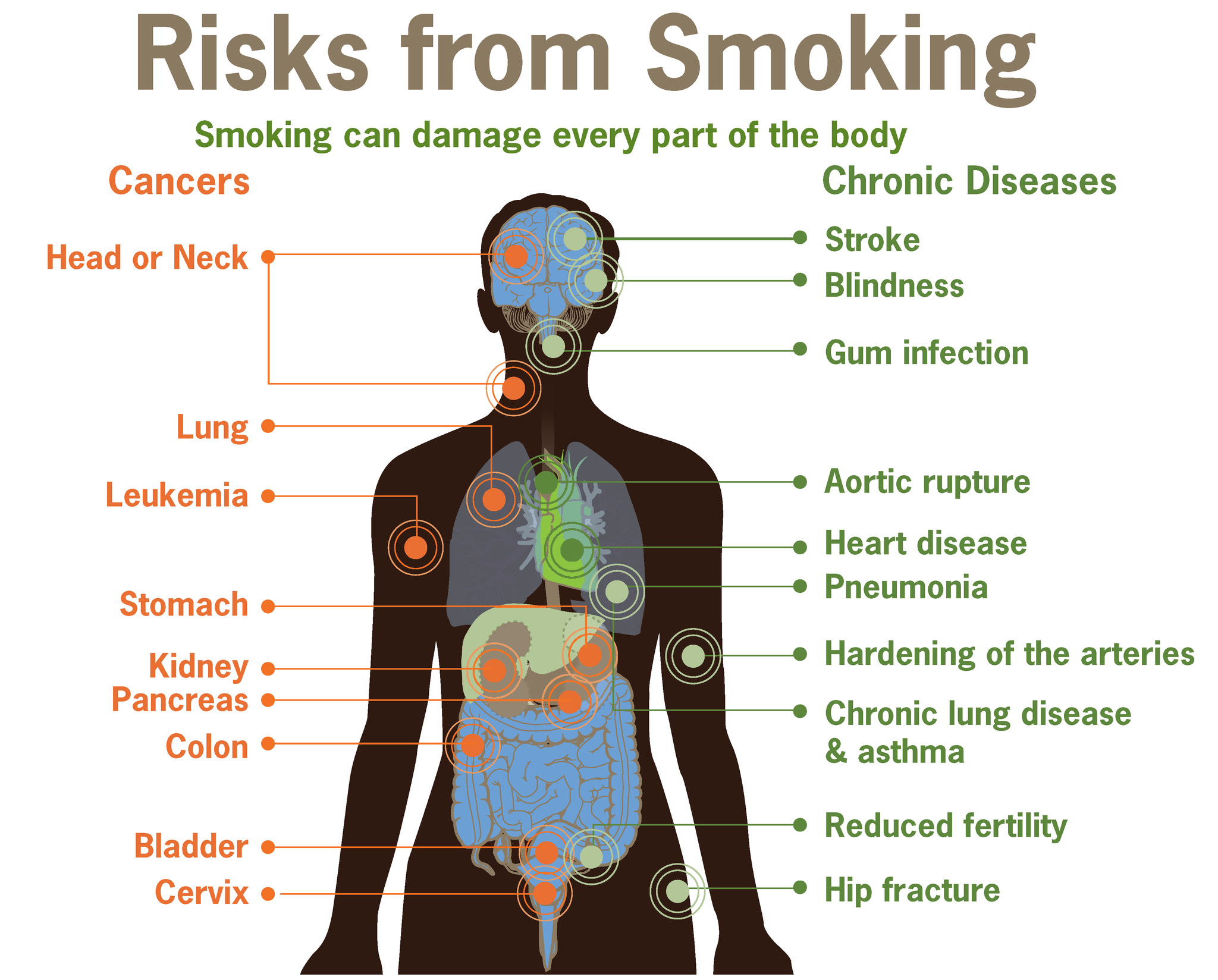
As shown in Figure 13.6.2, tobacco smoking has adverse effects on just about every bodily system and organ. The detrimental health effects of smoking depend on the number of years that a person smokes and how much the person smokes. Contrary to popular belief, all forms of tobacco smoke — including smoke from cigars and tobacco pipes — have similar health risks as those of cigarette smoke. Smokeless tobacco may be less of a danger to the lungs and heart, but it, too, has serious health effects. It significantly increases the risk of cancers of the mouth and throat, among other health problems.
Even non-smokers may not be spared the deadly risks of tobacco smoke. If you spend time around smokers either at home or on the job, then you are at risk of the dangers of secondhand smoke. Secondhand smoke enters the air directly from burning cigarettes (and cigars and pipes), and indirectly from smokers’ lungs. This smoke may linger in indoor air for hours, and it increases the risk of a wide range of adverse health effects. According to Health Canada, second-hand smoke causes 800 deaths from lung cancer and heart disease in non-smokers every year. The 2014 U.S. Surgeon General’s Report concluded that there is no established risk-free level of exposure to secondhand smoke. Non-smokers who are exposed to secondhand smoke may have as much as a 30 per cent increase in their risk of lung cancer and heart disease.
Tobacco contains nicotine, which is a psychoactive drug. Although nicotine in tobacco smoke does not directly cause cancer or most of the other health risks of smoking, it is a highly addictive drug. Nicotine is actually even more addictive than cocaine or heroin. The addictive nature of nicotine explains why it is so difficult for smokers to quit the habit, even when they know the health risks and really want to stop smoking. The good news is that if someone does stop smoking, his or her risks of smoking-related diseases and death soon start to fall. By one year after quitting, the risk of heart disease drops to only half of that of a continuing smoker.
Smoking and Cancer
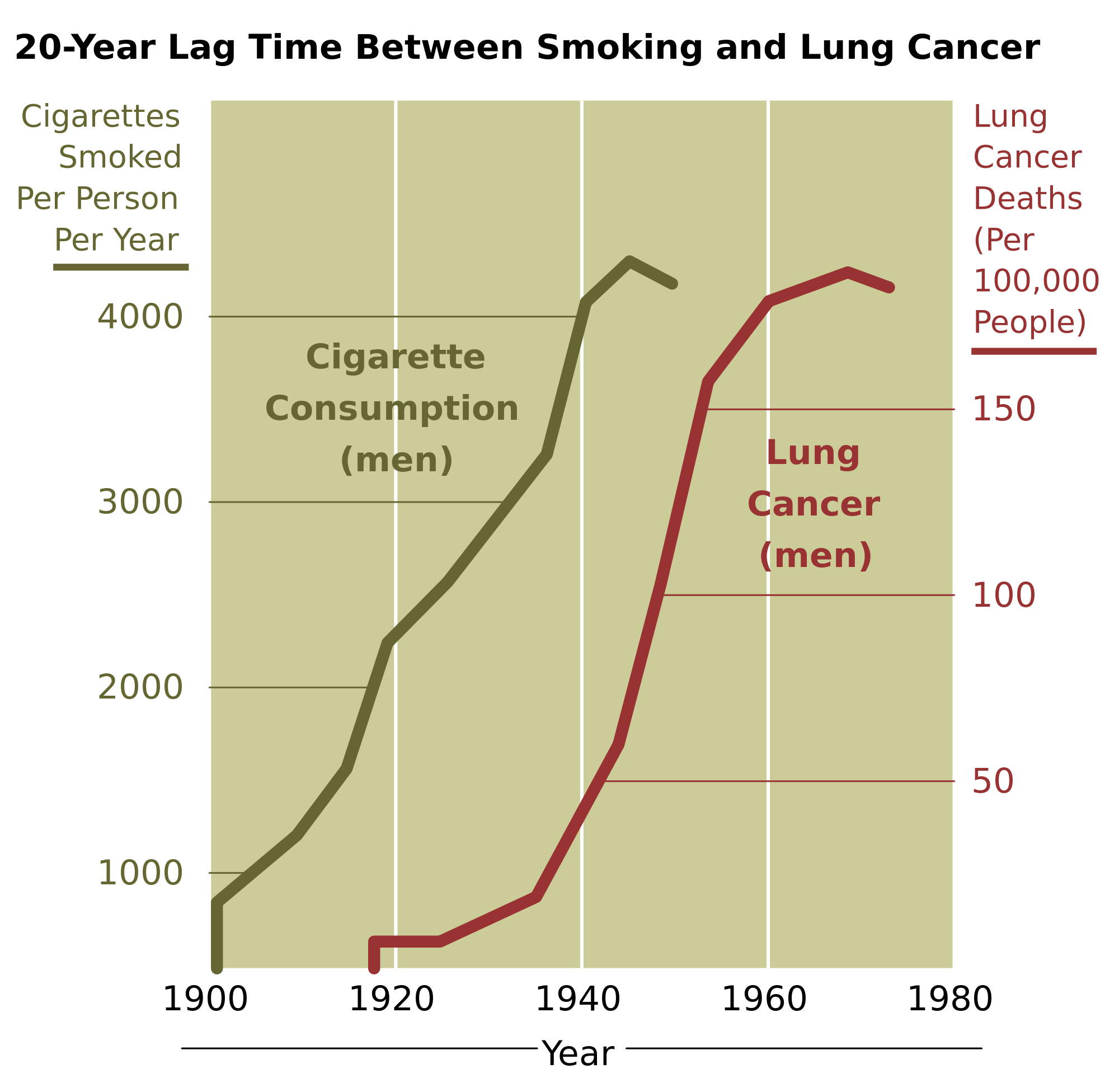
One of the main health risks of smoking is cancer, particular cancer of the lung. Because of the increased risk of lung cancer with smoking, the risk of dying from lung cancer before age 85 is more than 20 times higher for a male smoker than for a male non-smoker. As the rate of smoking increases, so does the rate of lung cancer deaths, although the effects of smoking on lung cancer deaths can take up to 20 years to manifest themselves, as shown in Figure 13.6.3. Besides lung cancer, several other forms of cancer are also significantly more likely in smokers than non-smokers, including cancers of the kidney, larynx, mouth, lip, tongue, throat, bladder, esophagus, pancreas, and stomach. Unfortunately, many of these cancers have extremely low cure rates.
When you consider the composition of tobacco smoke, it’s not surprising that it increases the risk of cancer. Tobacco smoke contains dozens of chemicals proven to be carcinogens, or causes of cancer. Many of these chemicals bind to DNA in a smoker’s cells, and may either kill the cells or cause mutations. If the mutations inhibit programmed cell death, the cells can survive to become cancer cells. Some of the most potent carcinogens in tobacco smoke include benzopyrene, acrolein, and nitrosamines. Other carcinogens in tobacco smoke are radioactive isotopes, including lead-210 and polonium-210.
Respiratory Effects of Smoking
Long-term exposure to the compounds found in cigarette smoke — such as carbon monoxide and cyanide — are thought to be responsible for much of the lung damage caused by smoking. These chemicals reduce the elasticity of alveoli, leading to chronic obstructive pulmonary disease (COPD). COPD is a permanent, incurable, and often fatal reduction in the capacity of the lungs, reducing the lungs’ ability to fully exhale air. The chronic inflammation that is also present in COPD is exacerbated by the tobacco smoke carcinogen acrolein and its derivatives. COPD is almost completely preventable simply by not smoking and by also avoiding secondhand smoke.
Cardiovascular Effects of Smoking
Inhalation of tobacco smoke causes several immediate responses in the heart and blood vessels. Within one minute of inhalation of smoke, the heart rate begins to rise, increasing by as much as 30 per cent during the first ten minutes of smoking. Carbon monoxide in tobacco smoke binds with hemoglobin in red blood cells, thereby reducing the blood’s ability to carry oxygen. Hemoglobin bound to carbon monoxide forms such a stable complex that it may result in a permanent loss of red blood cell function. Several other chemicals in tobacco smoke lead to narrowing and weakening of blood vessels, as well as an increase in substances that contribute to blood clotting. These changes increase blood pressure and the chances of a blood clot forming and blocking a vessel, thereby elevating the risk of heart attack and stroke. A recent study found that smokers are five times more likely than non-smokers to have a heart attack before the age of 40.
Smoking has also been shown to have a negative impact on levels of blood lipids. Total cholesterol levels tend to be higher in smokers than non-smokers. Ratios of “good” cholesterol to “bad” cholesterol tend to be lower in smokers than non-smokers.
Additional Adverse Health Effects of Smoking
A wide diversity of additional adverse health effects are attributable to smoking. Here are just a few of them:
- Smokers are at significantly increased risk of developing chronic kidney disease (in addition to kidney cancer). For example, smoking hastens the progression of kidney damage in people with diabetes.
- People who smoke — especially the elderly — have a greater risk of influenza and other infectious diseases than non-smokers. Smoking more than 20 cigarettes a day has been found to increase the risk of infectious diseases by as much as four times the risk in non-smokers. These effects occur because of damage to both the respiratory system and the immune system.
- In addition to oral cancer, smoking causes other oral problems, including periodontitis (gum disease). Roughly half of the cases of gum inflammation are attributable to current or former smoking. This inflammation increases the risk of tooth loss, which is also higher in smokers than non-smokers. In addition, smoking stains the teeth and causes halitosis (bad breath).
- Smoking is a key cause of erectile dysfunction (ED), probably because it leads to narrowing of arteries in the penis, as it does elsewhere in the body. The incidence of ED is about 85 per cent higher in males who smoke than it is in non-smokers.
- Smoking also has adverse effects on the female reproductive system, potentially causing infertility, in part because it interferes with the body’s ability to produce estrogen. Female smokers are about 60 per cent more likely to be infertile than non-smokers. Pregnant women who smoke or are exposed to secondhand smoke have a higher risk of miscarriages and low-birth-weight infants.
- Certain therapeutic drugs, including some antidepressants and anticonvulsants, are less effective in smokers than in non-smokers. This occurs because smoking increases levels of liver enzymes that break down the drugs.
- Smoking causes an estimated ten per cent of all fire-related deaths worldwide. Smokers are also at a greater risk of dying in motor vehicle crashes and other accidents.
- Smoking leads to an increased risk of bone fractures, especially of the hip. It also leads to slower wound healing after surgery, and an increased rate of postoperative complications.
Feature: Human Biology in the News

The item in Figure 13.6.4 looks like a regular cigarette, but it’s actually an electronic cigarette, or e-cigarette. E-cigarettes are battery-powered devices that change flavored liquids and nicotine into vapor that the user inhales. E-cigarettes are often promoted as being safer than traditional tobacco products, and their use is touted as a good way to quit smoking. They are often not banned in smoke-free areas, where it is illegal to smoke tobacco cigarettes.
A study completed in 2015 by researchers at the Harvard School of Public Health and widely reported in the mass media found that e-cigarettes may, in fact, be very harmful to the user’s health. E-cigarettes contain nicotine and cancer-causing chemicals, such as formaldehyde. According to the study, about three-quarters of flavored e-cigarettes also contain a chemical called diacetyl that causes an incurable and potentially fatal disorder of the lungs, commonly called “popcorn lung” (bronchiolitis obliterans). In this disorder, the bronchioles compress and narrow due to the formation of scar tissue, greatly diminishing the breathing capacity of people with the disorder. Popcorn lung gained its common name in 2004, when it was diagnosed in workers at popcorn factories. The buttery flavoring used in the factories contained diacetyl.
Some manufacturers of e-cigarettes and flavorings advertise that their products are now free of diacetyl. However, because e-cigarettes are not currently regulated by the FDA, there is no way of knowing for sure whether the products are actually safe. Equally disturbing is the appeal of flavored e-cigarettes to teens and producers’ attempts to specifically market their products to this age group. Flavors such as “cotton candy,” “Katy Perry’s cherry,” and “alien blood” are obviously marketed to youth. Not surprisingly, the use of e-cigarettes is on the rise in middle and high school students, who are more likely to use them than regular cigarettes. Public health officials fear that e-cigarettes will be a gateway for teens to move on to smoking tobacco cigarettes. Some U.S. states have recently passed laws prohibiting minors from buying e-cigarettes, and Brazil, Singapore, Uruguay, and India have banned e-cigarettes. E-cigarettes were not initially regulated by Health Canada because they don’t contain nicotine, which made them illegal to sell, but this was not widely enforced. However, Canada enacted the Tobacco and Vaping Products Act (TVPA) on May 23, 2018. As more questions are raised about their potential negative health effects, it is likely that more laws will be passed to regulate e-cigarettes. Watch the news for updates on this issue.
13.6 Summary
- Smoking is the single greatest cause of preventable death worldwide. It has adverse effects on just about every body system and organ. Tobacco smoke affects not only smokers, but also non-smokers who are exposed to secondhand smoke. The nicotine in tobacco is highly addictive, making it very difficult to quit smoking.
- A major health risk of smoking is cancer of the lungs. Smoking also increases the risk of many other types of cancer. Tobacco smoke contains dozens of chemicals known as carcinogens.
- Smoking is the primary cause of chronic obstructive pulmonary disease (COPD). Chemicals such as carbon monoxide and cyanide in tobacco smoke reduce the elasticity of alveoli so the lungs can no longer fully exhale air.
- Smoking damages the cardiovascular system and increases the risk of high blood pressure, blood clots, heart attack, and stroke. Smoking also has a negative impact on levels of blood lipids.
- A wide diversity of additional adverse health effects are attributable to smoking, such as erectile dysfunction, female infertility, and slow wound healing.
13.6 Review Questions
- Create a pamphlet aimed at informing teenagers about the dangers of smoking. Include information about numbers of deaths associated with smoking, life expectancy of smokers, and long term healthy effects of smoking and exposure to second-hand smoke. Include a section on the chemicals present in tobacco smoke and e-cigarettes and some of the adverse affects associated with these chemicals.
- What smoking-related factors determine how smoking affects a smoker’s health?
- What are the two sources of secondhand cigarette smoke? How does exposure to secondhand smoke affect non-smokers?
- Why is it so difficult for smokers to quit the habit? How is their health likely to be affected by quitting?
- Why does smoking cause cancer? List five types of cancer that are significantly more likely in smokers than non-smokers.
- Explain how smoking causes COPD.
- Do you think e-cigarettes can be addictive? Explain your reasoning.
13.6 Explore More
Blowing Smoke: The Lost Legacy of the Surgeon General’s Report | Alan Blum | TEDxTuscaloosa, TEDx Talks, 2015.
https://www.youtube.com/watch?v=QL2-EsjfiAU
The dangers of vaping, RWJ Barnabas Health, 2019.
Attributions
Figure 13.6.1
Cigarette by Uitbundig on Unsplash is used under the Unsplash License (https://unsplash.com/license)
Figure 13.6.2
Risks_from_smoking-smoking_can_damage_every_part_of_the_body by CDC on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 13.6.3
Cancer_smoking_lung_cancer_correlation_from_NIH.svg by Sakurambo on Wikimedia Commons is in the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 13.6.4
An_Electronic_Cigarette_(11359245033) by Lindsay Fox from Newport beach, United States on Wikimedia Commons is used under a CC BY 2.0 (https://creativecommons.org/licenses/by/2.0) license.
References
Allen, J. G., Flanigan, S. S., LeBlanc, M., Vallarino, J., MacNaughton, P., Stewart, J. H., Christiani, D.C. (2016, June 1). Flavoring chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored E-cigarettes [online article]. Environmental Health Perspectives, 124:733–739. https://doi.org/10.1289/ehp.1510185
Health Canada. (2015). Dangers of second-hand smoke [online article]. Government of Canada. https://www.canada.ca/en/health-canada/services/smoking-tobacco/avoid-second-hand-smoke/second-hand-smoke/dangers-second-hand-smoke.html
RWJ Barnabas Health. (2019, September 25). The dangers of vaping. YouTube. https://www.youtube.com/watch?v=QL2-EsjfiAU&feature=youtu.be
TEDx Talks. (2015, August 7). Blowing smoke: The lost legacy of the surgeon general’s report | Alan Blum | TEDxTuscaloosa. https://www.youtube.com/watch?v=lgVvnnnawvw&feature=youtu.be
Tobacco and Vaping Products Act. (2016, June 26). Government of Canada. https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/legislation/federal-laws/tobacco-act.html
Vaping, E-cigarettes to be regulated by Health Canada. (2016, November 22). CBC/Radio-Canada. HTTP://www.cbc.ca/news/health/vaping-health-canada-legislation-1.3862589
Wikipedia contributors. (2020, August 9). Regulation of electronic cigarettes. In Wikipedia. https://en.wikipedia.org/w/index.php?title=Regulation_of_electronic_cigarettes&oldid=972059825
Created by: CK-12/Adapted by Christine Miller
Protein Shake

Drinks like this shake contain a lot of protein. Muscle tissue consists mainly of protein, so such drinks are popular with people who want to build muscle. Making up muscles is just one of a plethora of functions of this amazingly diverse class of biochemicals.
What Are Proteins?
Proteins are a major class of biochemical compounds made up of small monomer molecules called amino acids. More than 20 different amino acids are typically found in the proteins of living things. Small proteins may contain just a few hundred amino acids, while large proteins may contain thousands.
Protein Structure
When amino acids bind together, they may form short chains of two or just a few amino acids. These short chains are called peptides. When amino acids form long chains, the chains are called polypeptides. A protein consists of one or more polypeptides.
Proteins may have up to four levels of structure, from primary to quaternary. As a result, they can have tremendous diversity. Here are some additional details about the levels of protein structure:
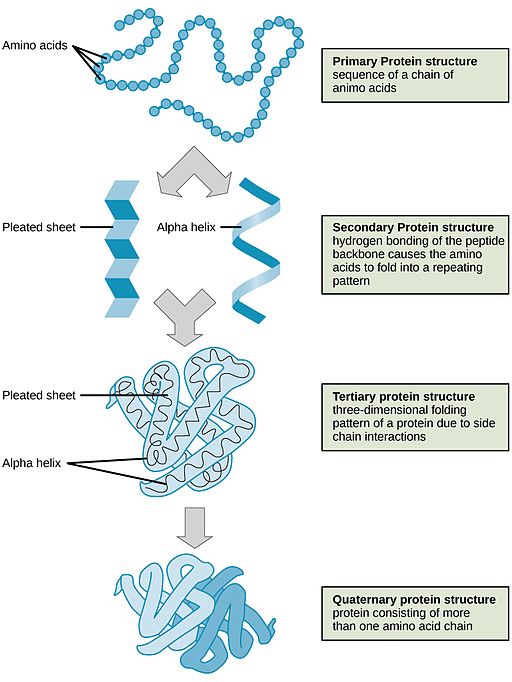
Figure 3.6.2 Four protein structures.
Functions of Proteins
The diversity of protein structures explains why this class of biochemical compounds can play so many important roles in living things. What are the roles of proteins?
- Some proteins have structural functions. They may help cells keep their shape or make up muscle tissues.
- Many proteins are enzymes that speed up chemical reactions in cells. Enzymes are usually highly specific and accelerate only one or a few chemical reactions. Thousands of different biochemical reactionsare known to be catalyzed by enzymes, including most of the reactions involved in metabolism. A reaction without an enzyme might take millions of years to complete, whereas with the proper enzyme, it may take just a few milliseconds!
- Other proteins are antibodies, which bind to specific foreign substances, like proteins on the surface of bacterial cells. This process targets the cells for destruction.
- Still other proteins carry messages or materials. Myoglobin, for example, is an oxygen-binding protein found in the muscle tissues of most mammals (including humans).
The chief characteristic of proteins that allows their diverse set of functions is their ability to bind to other molecules so specifically and tightly. Myoglobin can bind specifically and tightly with oxygen. The region of a protein responsible for binding with another molecule is known as the binding site. This site is often a depression on the molecular surface, determined largely by the tertiary structure of the protein.
Protein Consumption, Digestion, and Synthesis
Proteins are necessary in the diets of humans and other animals. We cannot make all the different amino acids we need, so we must obtain some of them from the foods we consume. In the process of digestion, we break down the proteins in food into free amino acids that can then be used to synthesize our own proteins. Protein synthesis from amino acid monomers takes place in all cells and is controlled by genes. Once new proteins are synthesized, they generally do not last very long before they are degraded and their amino acids are recycled. A protein's lifespan in mammalian cells is generally just a day or two.
3.6 Summary
- Proteins are a major class of biochemical compounds. They're made up of small monomer molecules called amino acids. More than 20 amino acids are commonly found in the proteins of living things. Proteins have tremendous diversity in terms of both structure and function.
- Long chains of amino acids form polypeptides. The sequence of amino acids in polypeptides makes up the primary structure of proteins. Proteins also have higher levels of structure. Secondary structure refers to configurations — such as helices and sheets — within polypeptide chains. Tertiary structure is a protein's overall three-dimensional shape, which controls the molecule's basic function. A quaternary structure forms if multiple protein molecules join together and function as a complex.
- Proteins help cells keep their shape, make up muscle tissues, act as enzymes or antibodies, and carry messages or materials. The chief characteristic that allows proteins' diverse functions is their ability to bind specifically and tightly with other molecules.
- We cannot make all the amino acids we need to synthesize our own proteins, so we must obtain some of them from proteins in the foods we consume.
3.6 Review Questions
- What are proteins?
- Outline the four levels of protein structure.
- Identify four functions of proteins.
- Explain why proteins can take on so many different functions in living things.
- What is the role of proteins in the human diet?
- Can you have a protein with both an alpha helix and a pleated sheet? Why or why not?
- If there is a mutation in a gene that causes a different amino acid to be encoded than the one usually encoded in that position within the protein, would that affect:
- The primary structure of the protein? Explain your answer.
- The higher structures (secondary, tertiary, quaternary) of the protein? Explain your answer.
- The function of the protein? Explain your answer.
- What is the region of a protein responsible for binding to another molecule? Which level or levels of protein structure creates this region?
- What is the region of a protein responsible for binding to another molecule? Which level or levels of protein structure creates this region?
-
- True or False: You can tell the function of all proteins based on their quaternary structure.
- Explain what the reading means when it says that amino acids are “recycled.”
3.6 Explore More
https://youtu.be/hok2hyED9go
Protein Structure and Folding by The Amoeba Sisters, 2018.
Attributions
Figure 3.6.1
Protein_shake by Sandstein, on Wikimedia Commons, is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 3.6.2
Structures of Protein by OpenStax, on Wikimedia Commons, is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
References
Amoeba Sisters. (2018, September 24). Protein structure and folding. YouTube. https://www.youtube.com/watch?v=hok2hyED9go
OpenStax. (2012, Aug 22). Figure 9. The four levels of protein structure can be observed in these illustrations. (credit: modification of work by National Human Genome Research Institute). In Biology. OpenStax CNX. © Rice University. https://cnx.org/contents/GFy_h8cu@10.53:2zzm1QG9@7/Proteins (last revised May 27, 2016).
A group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body.
Diagram shows examples of the shapes of different types of fatty acids. Saturated fatty acids form long straight chains. Monounsaturated fatty acids have a slight curve and saturated fatty acids can have multiple curves or bends.
Who's Who?
Figure 3.7.1 Identical twins show clearly the importance of genes in making us who we are. Genes would not be possible without nucleic acids.
What Are Nucleic Acids?
Nucleic acids are the class of biochemical compounds that includes DNA and RNA. These molecules are built of small monomers called nucleotides. Many nucleotides bind together to form a chain called a polynucleotide. The nucleic acid DNA (deoxyribonucleic acid) consists of two polynucleotide chains or strands. Thus, DNA is sometimes called double-stranded. The nucleic acid RNA (ribonucleic acid) consists of just one polynucleotide chain or strand, so RNA is sometimes called single-stranded.
Structure of Nucleic Acids
Each nucleotide consists of three smaller molecules:
- A sugar molecule (the sugar deoxyribose in DNA and the sugar ribose in RNA)
- A phosphate group
- A nitrogen base
The nitrogen bases in a nucleic acid stick out from the backbone. There are four different nitrogen bases: cytosine, adenine, guanine, and either thymine (in DNA) or uracil (in RNA). In DNA, bonds form between bases on the two nucleotide chains and hold the chains together. Each type of base binds with just one other type of base: cytosine always binds with guanine, and adenine always binds with thymine. These pairs of bases are called complementary base pairs.
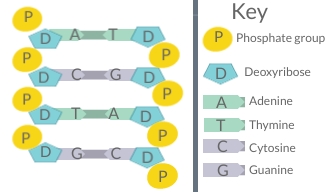
As you can see in Figure 3.7.2, sugars and phosphate groups form the backbone of a polynucleotide chain. Hydrogen bonds between complementary bases hold the two polynucleotide chains together.
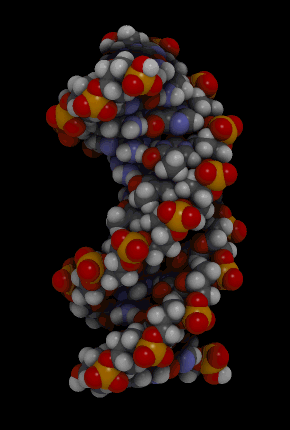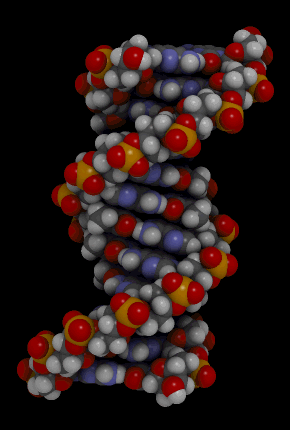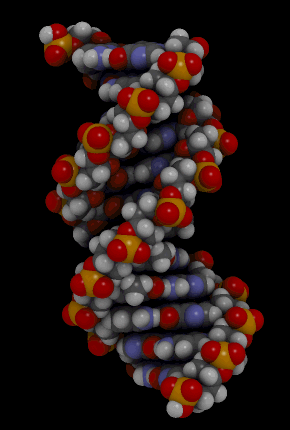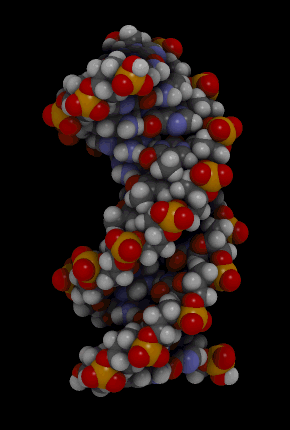
The binding of complementary bases causes DNA molecules automatically to take their well-known double helix shape, which is shown in the animation in Figure 3.7.3. A double helix is like a spiral staircase. It forms naturally and is very strong, making the two polynucleotide chains difficult to break apart.
DNA Molecule. Hydrogen bonds between complementary bases help form the double helix of a DNA molecule. The letters A, T, G, and C stand for the bases adenine, thymine, guanine, and cytosine. The sequence of these four bases in DNA is a code that carries instructions for making proteins. Shown is a representation of how the double helix folds into a chromosome.
Roles of Nucleic Acids
DNA makes up genes, and the sequence of bases in DNA makes up the genetic code. Between “starts” and “stops,” the code carries instructions for the correct sequence of amino acids in a protein. RNA uses the information in DNA to assemble the correct amino acids and help make the protein. The information in DNA is passed from parent cells to daughter cells whenever cells divide, and it is also passed from parents to offspring when organisms reproduce. This is how inherited characteristics are passed from one generation to the next.
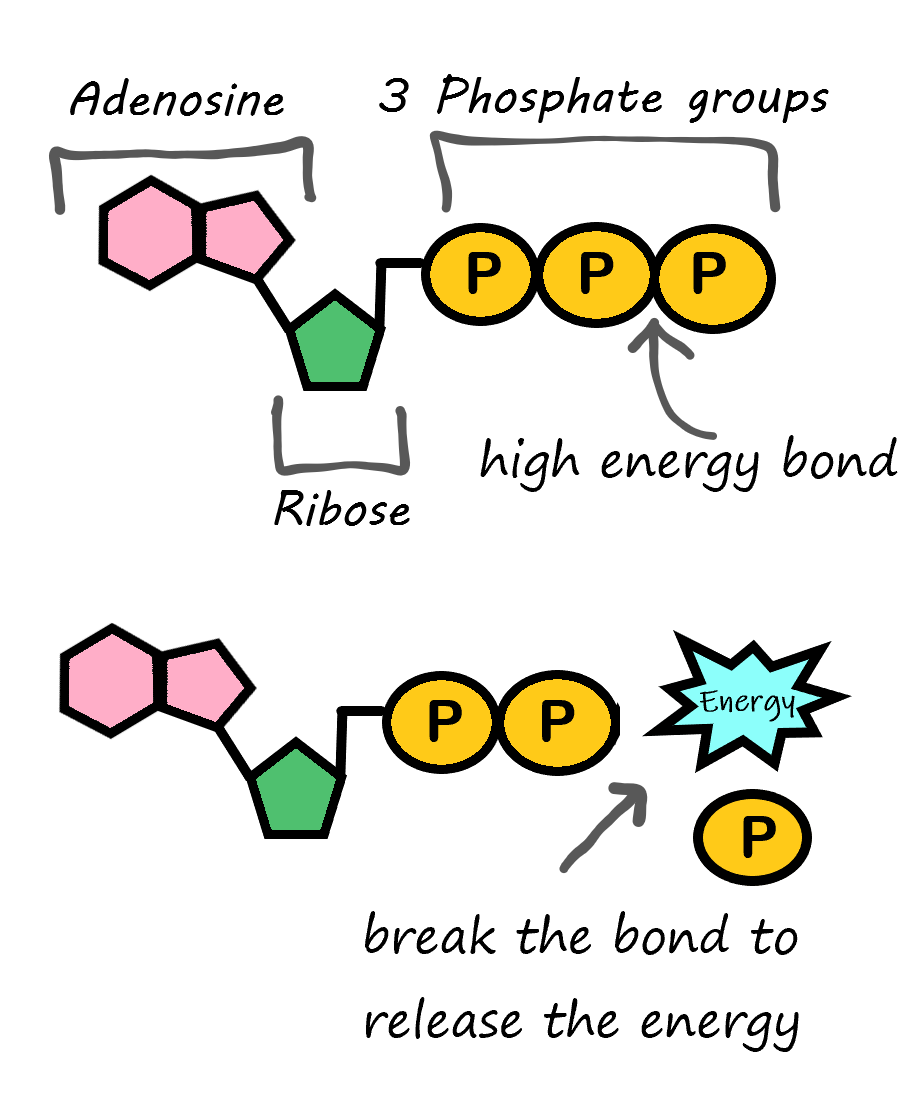
ATP is Energy
There is one type of specialized nucleic acid that exists only as a monomer. It stands apart from the other nucleic acids because it does not code for, or help create, proteins. This molecule is ATP, which stands for adenosine triphosphate. It consists of a sugar, adenosine, and three phosphate groups. It's primary role is as the basic energy currency in the cell. The way ATP works is all based on the phosphates. As shown in Figure 3.7.4, a large amount of energy is stored in the bond between the second and third phosphate group. When this bond is broken, it functions as an exothermic reaction and this energy can be used to power other processes taking place in the cell.
3.7 Summary
- Nucleic acids are the class of biochemical compounds that includes DNA and RNA. These molecules are built of small monomers called nucleotides, which bind together in long chains to form polynucleotides. DNA consists of two polynucleotides, and RNA consists of one polynucleotide.
- Each nucleotide consists of a sugar molecule, phosphate group, and nitrogen base. Sugars and phosphate groups of adjacent nucleotides bind together to form the "backbone" of the polynucleotide. Nitrogen bases jut out to the side of the sugar-phosphate backbone. Bonds between complementary bases hold together the two polynucleotide chains of DNA and cause it to take on its characteristic double helix shape.
- DNA makes up genes, and the sequence of nitrogen bases in DNA makes up the genetic code for the synthesis of proteins. RNA helps synthesize proteins in cells. The genetic code in DNA is also passed from parents to offspring during reproduction, which explains how inherited characteristics are passed from one generation to the next.
3.7 Review Questions
- What are nucleic acids?
- How does RNA differ structurally from DNA? Draw a picture of each.
- Describe a nucleotide. Explain how nucleotides bind together to form a polynucleotide.
- What role do nitrogen bases in nucleotides play in the structure and function of DNA?
- What is a function of RNA?
- Using what you learned in this article about nucleic acids, explain why twins look so similar.
-
- What are the nucleotides on the complementary strand of DNA below?
- Arrange the following in order from the smallest to the largest level of organization: DNA, nucleotide, polynucleotide.
- As part of the DNA replication process, the two polynucleotide chains are separated from each other, but each individual chain remains intact. What type of bonds are broken in this process?
- Adenine, guanine, cytosine, and thymine are _______________.
- Some diseases and disorders are caused by genes. Explain why these genetic disorders can be passed down from parents to their children.
- Are there any genetic disorders that run in your family?
3.7 Explore More
https://www.youtube.com/watch?v=aeAL6xThfL8
DNA: The book of you - Joe Hanson, TED-Ed, 2012.
Attributions
Figure 3.7.1
- Twins sitting next to each other by Craig Adderley on Pexels is used under the Pexels license (https://www.pexels.com/license/).
- Photograph Of Women Wearing Strip Shirt by Paul Bonafide Eferiano on Pexels is used under the Pexels license (https://www.pexels.com/license/).
- Two guys sitting on a beach by Daria Shevtsova on Pexels is used under the Pexels license (https://www.pexels.com/license/).
- Children Twins Girls Young Nicaraguan Portrait by skeeze on Pixabay is used under the Pixabay License (https://pixabay.com/service/license/).
Figure 3.7.2
DNA-diagram by Christine Miller [Christinelmiller] on Wikimedia Commons, is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
Figure 3.7.3
Bdna_cropped [gif] by Spiffistan, derivative work: Jahobr, on Wikimedia Commons, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 3.7.4
ATP for energy by Christine Miller is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) license.
Reference
TED-Ed. (2012, November 26). DNA: The book of you - Joe Hanson. YouTube, 2012. https://www.youtube.com/watch?v=aeAL6xThfL8&feature=youtu.be
Image shows a pictomicrograph of a protozoan parasite of the Giardia lamblia species. It is roughly cone-shaped, with several flagella trailing from the narrow end of it.
Refers to the body system consisting of the heart, blood vessels and the blood. Blood contains oxygen and other nutrients which your body needs to survive. The body takes these essential nutrients from the blood.

