43 5.2 Chemical Weathering — Physical Geology – 2nd Edition
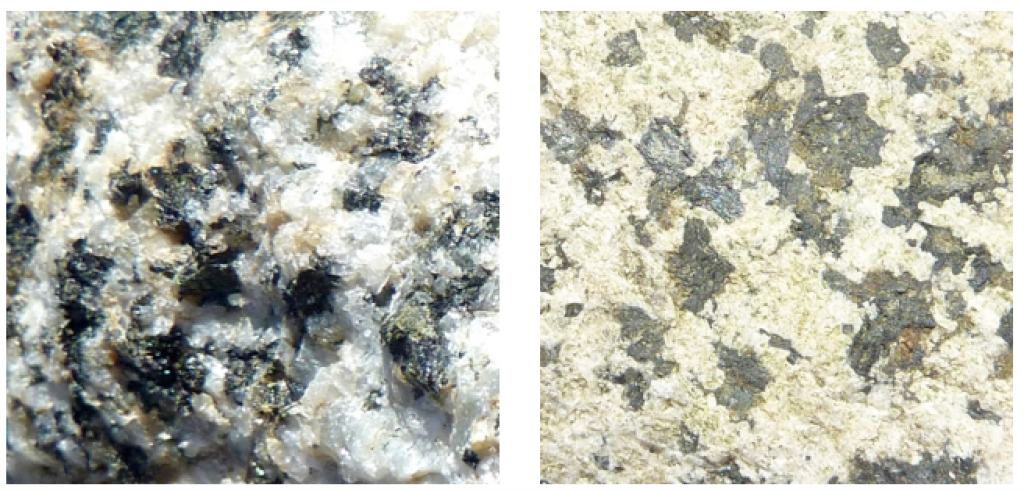
Figure 5.2.1 Unweathered (left) and weathered (right) surfaces of the same piece of granitic rock. On the unweathered surfaces the feldspars are still fresh and glassy-looking. On the weathered surface much of the feldspar has been altered to the chalky-looking clay mineral kaolinite.
Oxidation is another very important chemical weathering process. The oxidation of the iron in a ferromagnesian silicate starts with the dissolution of the iron. For olivine, the process looks like this, where olivine in the presence of carbonic acid is converted to dissolved iron, carbonate, and silicic acid:
|
But in the presence of oxygen and carbonic acid, the dissolved iron is then quickly converted to the mineral hematite:
|
The equation shown here is for olivine, but it could apply to almost any other ferromagnesian silicate, including pyroxene, amphibole, or biotite. Iron in the sulphide minerals (e.g., pyrite) can also be oxidized in this way. And the mineral hematite is not the only possible end result, as there is a wide range of iron oxide minerals that can form in this way. The results of this process are illustrated in Figure 5.2.2, which shows a granitic rock in which some of the biotite and amphibole have been altered to form the iron oxide mineral limonite.

Figure 5.2.2 A granitic rock containing biotite and amphibole which have been altered near to the rock’s surface to limonite, which is a mixture of iron oxide minerals.
A special type of oxidation takes place in areas where the rocks have elevated levels of sulphide minerals, especially pyrite (FeS2). Pyrite reacts with water and oxygen to form sulphuric acid, as follows:
|
The runoff from areas where this process is taking place is known as acid rock drainage (ARD), and even a rock with 1% or 2% pyrite can produce significant ARD. Some of the worst examples of ARD are at metal mine sites, especially where pyrite-bearing rock and waste material have been mined from deep underground and then piled up and left exposed to water and oxygen. One example of that is the Mt. Washington Mine near Courtenay on Vancouver Island (Figure 5.2.3), but there are many similar sites across Canada and around the world.

Figure 5.2.3 Exposed oxidizing and acid generating rocks and mine waste at the abandoned Mt. Washington Mine, B.C. (left), and an example of acid drainage downstream from the mine site (right).
At many ARD sites, the pH of the runoff water is less than 4 (very acidic). Under these conditions, metals such as copper, zinc, and lead are quite soluble, and this can lead to toxicity for aquatic and other organisms. For many years, the river downstream from the Mt. Washington Mine had so much dissolved copper in it that it was toxic to salmon. Remediation work has since been carried out at the mine and the situation has improved.
The hydrolysis of feldspar and other silicate minerals and the oxidation of iron in ferromagnesian silicates all serve to create rocks that are softer and weaker than they were to begin with, and thus more susceptible to mechanical weathering.
The weathering reactions that we’ve discussed so far involved the transformation of one mineral to another mineral (e.g., feldspar to clay), and the release of some ions in solution (e.g., Ca2+ or Fe2+). Some weathering processes involve the complete dissolution of a mineral. Calcite, for example, will dissolve in weak acid, to produce calcium and bicarbonate ions. The equation is as follows:
|
Calcite is the major component of limestone (typically more than 95%), and under surface conditions, limestone can dissolve completely, as shown in Figure 5.2.4. Limestone also dissolves at relatively shallow depths underground, forming limestone caves. This is discussed in more detail in Chapter 14, where we look at groundwater.

Figure 5.2.4 A limestone outcrop on Quadra Island, B.C. The limestone, which is primarily made up of the mineral calcite, has been dissolved to different degrees in different areas because of compositional differences. The buff-coloured bands are chert, which stands out because it is not soluble.
Media Attributions
- Figure 5.2.1, 5.2.2, 5.2.3, 5.2.4: © Steven Earle. CC BY.
<!– pb_fixme –>
|
|
|
|
<!– pb_fixme –>
<!– pb_fixme –>





