12.4 Hydrogenous Sediments
Methane hydrate section modified from "Physical Geology" by Steven Earle*
Paul Webb
As we saw in section 5.3 seawater contains many different dissolved substances. Occasionally chemical reactions occur that cause these substances to precipitate out as solid particles, which then accumulate as hydrogenous sediment. These reactions are usually triggered by a change in conditions, such as a change in temperature, pressure, or pH, which reduces the amount of a substance that can remain in a dissolved state. There is not a lot of hydrogenous sediment in the ocean compared to lithogenous or biogenous sediments, but there are some interesting forms.
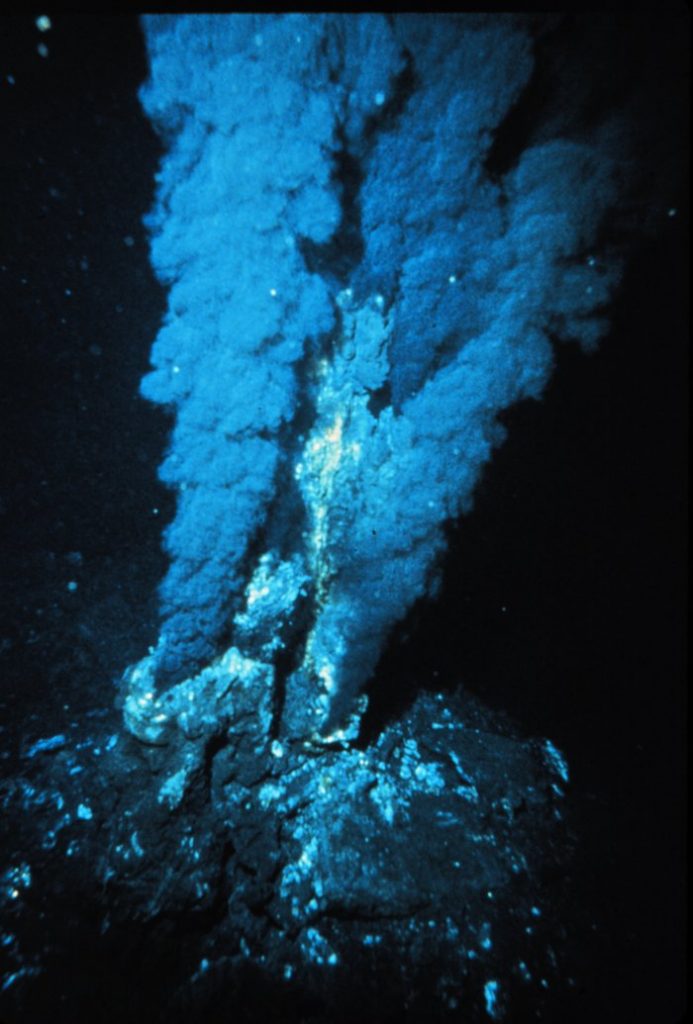
Hydrothermal vents were discussed in section 4.11. Recall that in these systems, seawater percolates into the seafloor, where it becomes superheated by magma before being expelled by the vent. This superheated water contains many dissolved substances, and when it encounters the cold seawater after leaving the vent, these particles precipitate out, mostly as metal sulfides. These particles make up the “smoke” that flows from a vent, and may eventually settle on the bottom as hydrogenous sediment (Figure 12.4.1).
Manganese nodules are rounded lumps of manganese and other metals that form on the seafloor, generally ranging between 3-10 cm in diameter, although they may sometimes reach up to 30 cm (Figure 12.4.2). The nodules form in a manner similar to pearls; there is a central object around which concentric layers are slowly deposited, causing the nodule to grow over time. The composition of the nodules can vary somewhat depending on their location and the conditions of their formation, but they are usually dominated by manganese- and iron oxides. They may also contain smaller amounts of other metals such as copper, nickel and cobalt. The precipitation of manganese nodules is one of the slowest geological processes known; they grow on the order of a few millimeters per million years. For that reason, they only form in areas where there are low rates of lithogenous or biogenous sediment accumulation, because any other sediment deposition would quickly cover the nodules and prevent further nodule growth. Therefore, manganese nodules are usually limited to areas in the central ocean, far from significant lithogenous or biogenous inputs, where they can sometimes accumulate in large numbers on the seafloor (Figure 12.4.2 right). Because the nodules contain a number of commercially valuable metals, there has been significant interest in mining the nodules over the last several decades, although most of the efforts have thus far remained at the exploratory stage. A number of factors have prevented large-scale extraction of nodules, including the high costs of deep sea mining operations, political issues over mining rights, and environmental concerns surrounding the extraction of these non-renewable resources.

Evaporites are hydrogenous sediments that form when seawater evaporates, leaving the dissolved materials to precipitate into solids, particularly halite (salt, NaCl). In fact, the evaporation of seawater is the oldest form of salt production for human use, and is still carried out today. Large deposits of halite evaporites exist in a number of places, including under the Mediterranean Sea. Beginning around 6 million years ago, tectonic processes closed off the Mediterranean Sea from the Atlantic, and the warm climate evaporated so much water that the Mediterranean was almost completely dried out, leaving large deposits of salt in its place (an event known as the Messinian Salinity Crisis). Eventually the Mediterranean re-flooded about 5.3 million years ago, and the halite deposits were covered by other sediments, but they still remain beneath the seafloor.

Oolites are small, rounded grains formed from concentric layers of precipitation of material around a suspended particle. They are usually composed of calcium carbonate, but they may also from from phosphates and other materials. Accumulation of oolites results in oolitic sand, which is found in its greatest abundance in the Bahamas (Figure 12.4.4).

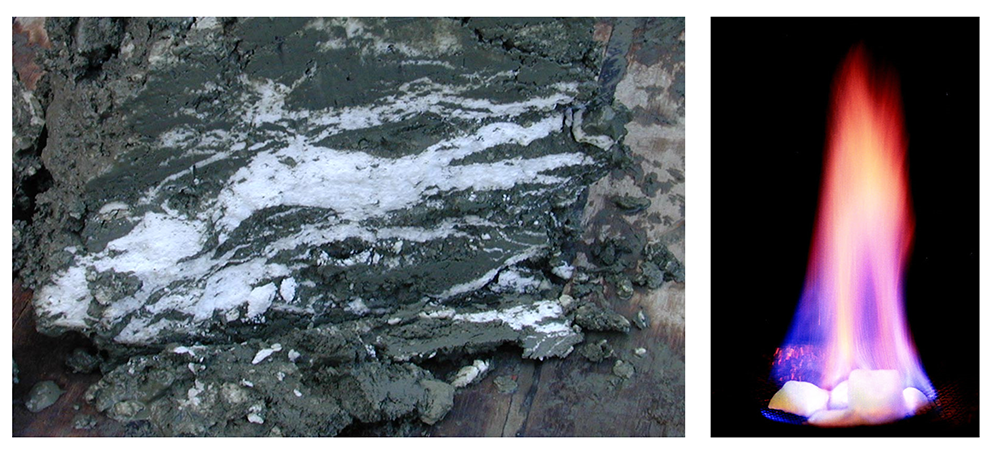
Methane hydrates are another type of hydrogenous deposit with a potential industrial application. All terrestrial erosion products include a small proportion of organic matter derived mostly from terrestrial plants. Tiny fragments of this material plus other organic matter from marine plants and animals accumulate in terrigenous sediments, especially within a few hundred kilometers of shore. As the sediments pile up, the deeper parts start to warm up (from geothermal heat), and bacteria get to work breaking down the contained organic matter. Because this is happening in the absence of oxygen (a.k.a. anaerobic conditions), the by-product of this metabolism is the gas methane (CH4). Methane released by the bacteria slowly bubbles upward through the sediment toward the seafloor. At water depths of 500 m to 1,000 m, and at the low temperatures typical of the seafloor (close to 4°C), water and methane combine to create a substance known as methane hydrate. Within a few meters to hundreds of meters of the seafloor, the temperature is low enough for methane hydrate to be stable and hydrates accumulate within the sediment (Figure 12.4.5 left). Methane hydrate is flammable because when it is heated, the methane is released as a gas (Figure 12.4.5 right). The methane within seafloor sediments represents an enormous reservoir of fossil fuel energy. Although energy corporations and governments are anxious to develop ways to produce and sell this methane, anyone that understands the climate-change implications of its extraction and use can see that this would be folly.
*”Physical Geology” by Steven Earle used under a CC-BY 4.0 international license. Download this book for free at http://open.bccampus.ca
sediments formed from the precipitation of dissolved substances (12.4)
sediment derived from preexisting rock (12.2)
sediment created from the remains of organisms (12.3)
area of the seafloor where superheated water seeps out of the crust (4.11)
molten rock typically dominated by silica (3.2)
spherical accumulations of manganese and other metals that form slowly through precipitation on the seafloor (12.4)
hydrogenous sediments that form when seawater evaporates (12.4)
a small (approximately 1 mm) sphere of calcite formed in areas of tropical shallow marine water (12.4)
a combination of water ice and methane in which the methane is trapped inside “cages” in the ice (12.4)
referring to sedimentary particles that originated on a continent (12.2)

