106 14.4 Groundwater Quality — Physical Geology – 2nd Edition
14.4 Groundwater Quality
As was noted at the very beginning of this chapter, one of the good things about groundwater as a source of water is that it is not as easily contaminated as surface water is. But there are two caveats to that: one is that groundwater can become naturally contaminated because of its very close connection to the materials of its aquifer, and the second is that once contaminated by human activities on surface, groundwater is very difficult to clean up.
Natural Contamination of Groundwater
Groundwater moves slowly through an aquifer, and unlike the surface water of a stream, it has a lot of contact with the surrounding rock or sediment. In most aquifers, the geological materials that make up the aquifer are relatively inert, or are made up of minerals that dissolve very slowly into the groundwater. Over time, however, all groundwater gradually has more and more material dissolved within it as it remains in contact with the aquifer. In some areas, that rock or sediment includes some minerals that could potentially contaminate the water with elements that might make the water less than ideal for human consumption or agricultural use. Examples include copper, arsenic, mercury, fluorine, sodium, and boron. In some cases, contamination may occur because the aquifer material has particularly high levels of the element in question. In other cases, the aquifer material is just normal rock or sediment, but some particular feature of the water or the aquifer allows the contaminant to build up to significant levels.
An example of natural contamination takes place in the Upper Cretaceous Mancos Shale (Figure 14.4.1). The shale has elevated concentrations of the element selenium (Se) in certain zones. Selenium in trace amounts is an essential micronutrient in all organisms, but is extremely toxic in elevated concentrations. Anomalously high selenium contents is found within the shale at the Middle Arkansas River Basin and the Gunnison River Basin. The shale also runs through the South Platte River Basin, but Se contents are not at hazardous levels there.
Another example of natural groundwater contamination comes with elevated salinity when water flows over and through ancient salt (evaporite) deposits (Figure 14.4.2.). Elevated salts in groundwater can negatively impact the growth of certain crops in agriculture.


One more general note relates to rocks that contain relatively high levels of clay (in which water can easily flow through such as a clay-cemented sandstone). The clay is good at adsorbing[1] some elements from the water and desorbing others, and in the process, its pH goes up (it becomes alkaline). At high pH levels (some as high as 9 in areas), the element fluorine that is naturally present in the rock (as it is in almost any rock) has an increased tendency to dissolve in the water. In some areas, groundwater has fluorine levels that are well above recommended levels for drinking water. The World Health Organization (WHO) maximum acceptable concentration (MAC) for fluorine is 1.5 milligrams per liter (milligrams per liter). Some domestic wells have recorded as much as 10 milligrams per liter! A small amount of fluorine in the human diet is considered important for maintaining dental health, but high levels can lead to malformation and discoloration of teeth, and long-term exposure can lead to other more serious health effects such as skeletal problems.
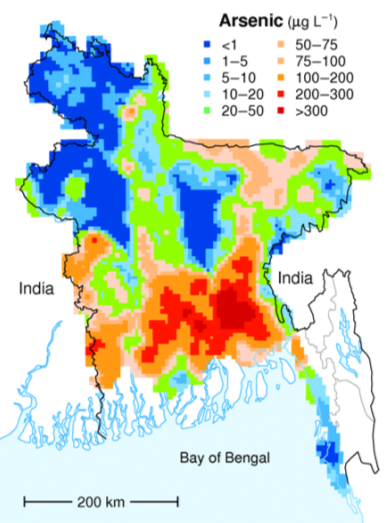
Rural residents in the densely populated country of Bangladesh (over 1,000 residents per square kilometer, compared with 19.9 per square kilometer in Colorado) used to rely mostly on surface supplies for their drinking water, and many of these were subject to bacterial contamination. Infant mortality rates were among the highest in the world and other illnesses such as diarrhea, dysentery, typhoid, cholera, and hepatitis were common. In the 1970s, international agencies, including UNICEF, started a program of drilling wells to access abundant groundwater supplies at depths of 20 meters to 100 meters. Eventually over 8 million such wells were drilled. Infant mortality and illness rates dropped dramatically, but it was later discovered that the water from a high proportion of these wells has arsenic above safe levels (Figure 14.4.3).
Most of the wells in the affected areas are drilled into relatively recent sediments of the vast delta of the Ganges and Brahmaputra Rivers. While these sediments are not particularly enriched in arsenic, they have enough organic matter in them to use up any oxygen present. This leads to water with a naturally low oxidation potential (anoxic conditions); arsenic is highly soluble under these conditions, and so any arsenic present in the sediments easily gets dissolved into the groundwater. Arsenic poisoning leads to headaches, confusion, and diarrhea, and eventually to vomiting, stomach pain, and convulsions. If not treated, the final outcomes are heart disease, stroke, cancer, diabetes, coma, and death. There are ways to treat arsenic-rich groundwater, but it is a challenge in Bangladesh to implement the simple and effective technology that is available.
Anthropogenic Contamination of Groundwater
Groundwater can become contaminated by pollution at the surface (or at depth), and there are many different anthropogenic (human-caused) sources of contamination.
The vulnerability of aquifers to pollution depends on several factors, including the depth to the water table, the permeability of the material between the surface and the aquifer, the permeability of the aquifer, the slope of the surface, and the amount of precipitation. Confined aquifers tend to be much less vulnerable than unconfined ones, and deeper aquifers are less vulnerable than shallow ones. Steeper slopes mean that surface water tends to run off rather than infiltrate (and this can reduce the possibility of contamination). Contamination risk is also less in dry areas than in areas with heavy rainfall.
One of the most significant examples of human-caused contamination in Colorado is in the Denver-Julesburg Basin in northeastern Colorado. The area is dotted with oil and natural gas wells, and has been a site with frequent hydraulic fracturing (fracking) activity. A study conducted by the University of Colorado has shown that the elevated concentrations of methane in groundwater there has not improv
The important sources of anthropogenic groundwater contamination include the following:
- Chemicals and animal waste related to agriculture, and chemicals applied to golf courses and domestic gardens
- Landfills
- Industrial operations
- Mines, quarries, and other rock excavations
- Leaking fuel storage tanks (especially those at gas stations)
- Septic systems
- Runoff from roads (e.g., winter salting) or chemical spills of materials being transported
Agriculture
Intensive agricultural operations and golf courses can have a significant impact on the environment, especially where chemicals and other materials are used to enhance growth or control pests. An example of agricultural contamination is in the Abbotsford area of the Fraser Valley, where nitrate levels above the 44 milligrams per liter maximum acceptable level (expressed as nitrate) in the Abbotsford-Sumas aquifer have been observed since the 1950s; however, the problem became much worse as agriculture intensity increased in the 1980s. By 2004, groundwater with nitrate levels in excess of 44 milligrams per liter was reported over an area of about 75 kilometers square around Abbotsford, and the problem extended across the border into the Sumas area of Washington State.
This region is intensively used for berry crops (especially raspberries and blueberries) and large poultry operations, as well as lesser amounts of grazing and forage crops. Chicken manure is typically stored in fields adjacent to chicken barns, and may release nitrogen to the environment from runoff water, and from releases of ammonia gas. Over decades, both chemical fertilizers and chicken manure and other manures have been applied to the berry crops to provide extra nitrogen to help maximize berry growth. If the fertilizer added is in excess of what the plants need, or is poorly timed compared to when it is needed, then the extra nitrogen may be leached into the groundwater below. Berry crops are irrigated over the summer to help the crops grow. Summer irrigation and winter rainfall may carry excess nitrate from the near surface to the aquifer below.
Since the 1990s, agricultural practices have been tightened up to reduce the rate of groundwater contamination, but it will take decades for nitrate levels to drop in the Abbotsford-Sumas aquifer. Agriculture and Agri-Food Canada and many others are conducting research on better irrigation and nitrate management techniques to reduce the amount of nitrogen that leaches to groundwater.
Landfills
In the past, domestic and commercial refuse was commonly trucked to a “dump” (typically a hole in the ground), and when the hole was filled, it was covered with soil and forgotten. In situations like this, rain and melting snow can easily pass through the soil used to cover the refuse. This water passes into the waste itself, and the resulting landfill leachate that flows from the bottom of the landfill can seriously contaminate the surrounding groundwater and surface water. In the past few decades, regulations around refuse disposal have been significantly strengthened, and important steps have been taken to reduce the amount of landfill waste by diverting recyclable and compostable materials to other locations.
A modern engineered landfill has an impermeable liner (typically heavy plastic, although engineered clay liners or natural clay may be adequate in some cases), a plumbing system for draining leachate (the rainwater that flows through the refuse and becomes contaminated), and a network of monitoring wells both within and around the landfill (Figure 14.4.4). Once part or all of a landfill site is full, it is sealed over with a plastic cover, and a system is put in place to extract landfill gas (typically a mixture of carbon dioxide and methane). That gas can be sent to a nearby location where it is burned to create heat or used to generate electricity. The leachate must be treated, and that can be done in a normal sewage treatment plant.
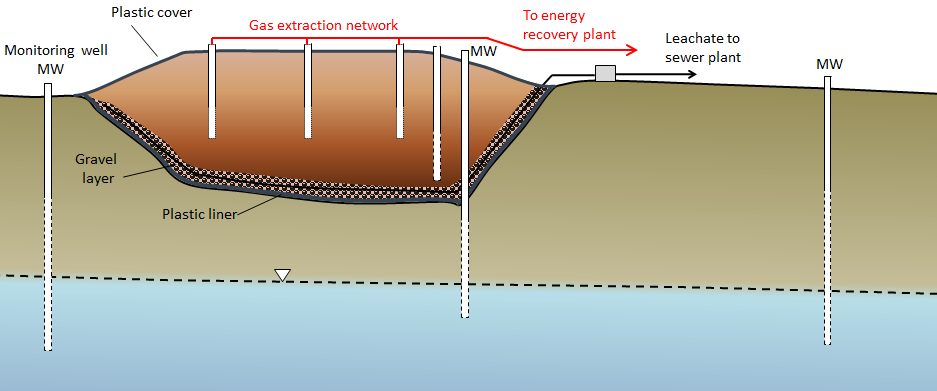
The monitoring wells are used to assess the level of the water table around the landfill and to collect groundwater samples so that any leakage can be detected. Because some leakage is almost inevitable, the ideal placement for landfills is in areas where the depth to the water table is significant (tens of meters if possible) and where the aquifer material is relatively impermeable. Landfills should also be situated far from streams, lakes, or wetlands so that contamination of aquatic habitats can be avoided.
Today there are hundreds of abandoned dumps scattered across the country; most have been left to contaminate groundwater that we might wish to use sometime in the future. In many cases, it’s unlikely that we’ll be able to do so.
An example of a landfill causing groundwater contamination, that has since turned positive is the Lowry Landfill, located in Aurora, CO. The 507-acre site started operations in the mid-1960s and took in both residential and industrial solid waster well into the 1980s. Improper storage practices contributed to harmful release of gases, most notably methane (CH4), into the soil and groundwater. The site was deemed a Superfund site in 1984. Various EPA cleanup activities included installation of a liner, a water treatment plant, and most notably a system that collects landfill gas and converts it to electricity. The generated power is enough to power 3,000 houses, which equates to an annual extraction of 5,000 tons of methane. Although dumped of new refuge has long-ended, there is still waste on the site. Due to this, the Superfund site must go through EPA site reviews every five years to ensure no new waste has spread beyond its boundaries. A summer 2021 report states that the site poses no risk to the health of nearby citizens.
Unless you live in a remote rural area, there’s a good chance that the refuse you can’t recycle is picked up at the curb and taken to a landfill. Most landfills are operated by cities or districts, and you should be able to find information about yours on the appropriate local government website. See if you can answer some the following questions:
- Which government body operates your landfill?
- Where is the landfill situated?
- Is your waste all placed in a landfill, or are there other processes in use (e.g., incineration or composting)?
- Are landfill gases captured, and, if so, what is done with them?
- What could be changed to improve the waste disposal situation in your community (e.g., more recycling, compost collection, waste-to-energy technology)?
See Appendix 3 for Exercise 14.4 answers.
Industrial Operations

Colorado contains multiple instances of groundwater contamination via industrial operations, in both urban and rural settings. One example is the Colorado Smelter, located in Pueblo. The facility refined silver and lead from the many mines dotting the Colorado Rockies starting in 1883, and operated for 25 years. Despite being closed for more than a century, the EPA declared the are a Superfund site due to elevated levels of lead and arsenic still within the soil and groundwater there (350 ppm and 61 ppm, respectively). The contamination has affected over 1,900 houses and commercial properties, all within a half-mile of the smelter.
Mines, Quarries, and Rock Excavations
Mines and other operations that involve the excavation of large amounts of rock (e.g., highway construction) have the potential to create serious environmental damage. The exposure of rock that has previously not been exposed to air and water can lead to the oxidation of sulfide-bearing minerals, such a pyrite (FeS2), within the rock. The combination of pyrite, water, oxygen, and a special type of bacteria (Acidithiobacillus ferrooxidans) that thrives in acidic conditions leads to the generation of acidity, in some cases to pH less than 2. Water that acidic is hazardous by itself, but the low pH also has the property of increasing the solubility of certain heavy metals. The water that is generated by this process is known as acid rock drainage (ARD). ARD can occur naturally where sulfide-bearing rocks are near the surface.

Figure 14.4.7 Acidic runoff at the abandoned Mt. Washington Mine near Courtenay, B.C. The issue of ARD is a major environmental concern at both operating mines and abandoned mines (see Chapter 20). In streams around the Mt. Washington Mine on Vancouver Island (Figure 14.4.7), copper levels are high enough to be toxic to fish. Groundwater adjacent to the contaminated streams in the area is very likely contaminated as well.
Leaking Fuel Tanks

Underground storage tanks (USTs) are used to store fuel at gas stations, industrial sites, airports, and anywhere that large volumes of fuel are used. They do not last forever, and eventually they start to leak their contents into the ground. This is a particular problem at older gas stations — although it may also become a future problem at newer gas stations. You may have noticed gas stations that have been closed and then surrounded by chain-link fence (Figure 14.4.8). In virtually all such cases the closure has been triggered by the discovery of leaking USTs and the requirement to cease operations and remediate the site.
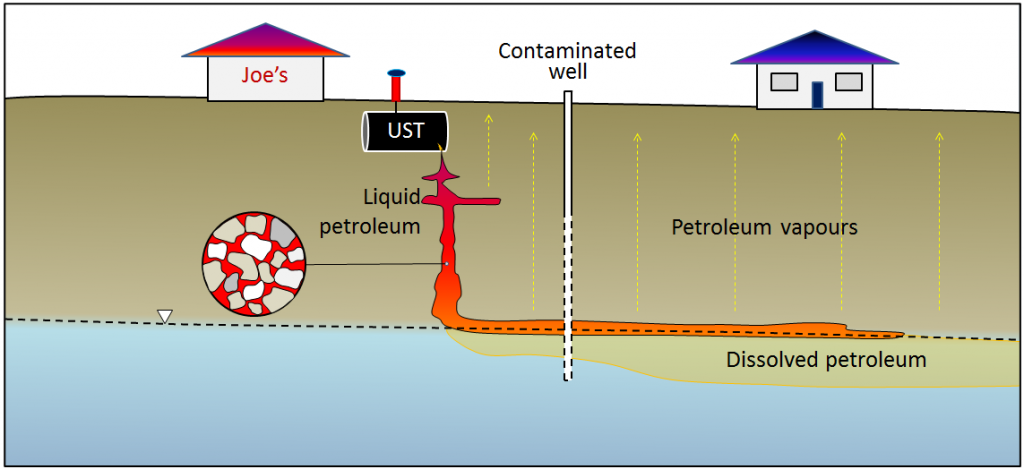
Petroleum fuels are complex mixtures of hydrocarbon compounds and the properties of their components—such as density, viscosity, solubility in water, and volatility—tend to vary widely. As a result, a petroleum spill is like several spills for the price of one. The petroleum liquid slowly settles through the unsaturated zone and then tends to float on the surface of the groundwater (Figure 14.4.9). The more readily soluble components of the spill dissolve in the groundwater and are dispersed along with the normal groundwater flow, and the more volatile components of the spill rise toward the surface, potentially contaminating buildings.
There is almost certainly a leaking UST at a former gas station near you. Look for an empty property that is surrounded by a chain-link fence with “No Trespassing” signs. You might see evidence of monitoring wells (like those shown in Figure 14.4.9, and there could be some petroleum barrels around that are being used to store contaminated water. Once you’ve identified one of these, you’ll probably start seeing them everywhere!
See Appendix 3 for Exercise 14.5 answers.
Septic Systems
In areas that are not served by sewage networks leading to a central sewage treatment plant, most homeowners rely on septic systems for disposal of sewage. There are two primary components to a simple septic system, the septic tank and the drainage field (Figure 14.4.10). A typical septic tank is constructed of either concrete or plastic and has a volume of 5,000 liters to 10,000 liters (5 m3 to 10 m3). This forms the first treatment and is designed to be anaerobic (without oxygen). That promotes the activity of certain bacteria that help break down the waste. As the waste is degraded, some portions tend to sink to form sludge at the base of the tank, and others float to the surface, forming a scum layer. A septic tank may be divided into two parts to keep the sludge at the bottom and the scum on the top from draining out. The water then moves to the drainage field, which provides the right conditions for a different set of bacteria that operate in aerobic conditions. The drainage field includes an array of plastic pipes that are perforated to allow the effluent to drain out over a large area and seep slowly into the ground. In order to install a drainage field, it is first necessary to test the soil below, as it must be sufficiently permeable to allow the effluent to percolate away, but not so permeable that it flows too quickly and the soil is not able to filter out the pathogenic bacteria.
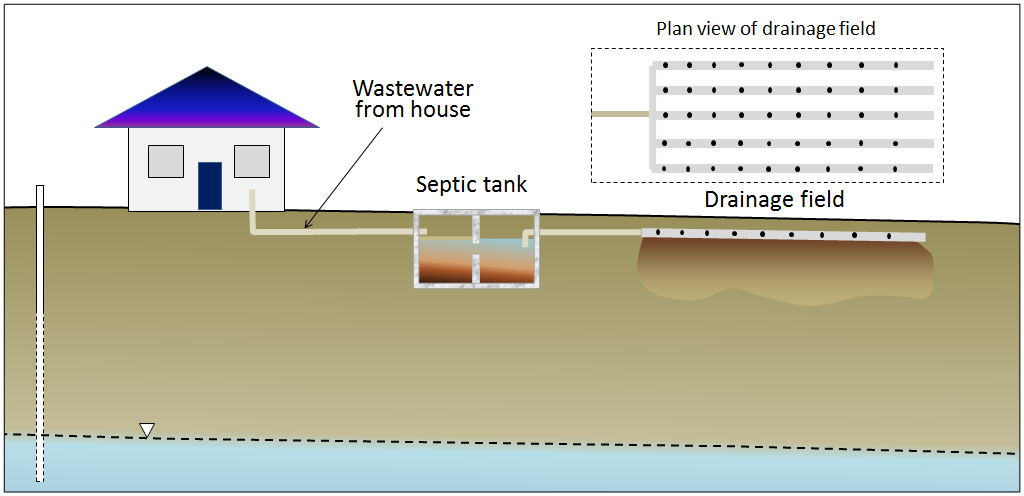
If they are properly installed and used, and if the sludge is periodically removed from the tank, a septic system should be effective in treating the sewage for decades. The anaerobic and aerobic bacteria should be able to break down the incoming waste and there should be little risk to the surface environment or groundwater. But many things can go wrong with a septic system, including the following:
- If inappropriate chemicals are added to the waste stream, they may interfere with the natural breakdown of the sewage.
- If the tank is not periodically pumped out, solids can get into the drainage field and compromise the drainage, resulting in the flow of effluent toward the surface.
- If the soil is either not sufficiently permeable or too permeable, the effluent will not drain away (and will start to pool at the surface) or it will drain too quickly.
- If the drainage field is constructed in an area where the water table is close to surface, some of the effluent is likely to flow into the groundwater without being treated.
Prevention and Mitigation of Groundwater Contamination
As illustrated in the landfill example above, there are two fairly simple ways to significantly reduce the chance and degree of groundwater contamination from surface sources. One is to prevent rainwater from infiltrating down to the water table and picking up contaminants; this can be achieved by simply capping or roofing over the landfill, mine tailings, or spill site. The second is to provide an impermeable barrier beneath the contaminant. Modern landfills and mine tailings impoundments are all built using some combination of clay and engineered plastic barriers. Both of these solutions — caps and liners — are subject to failure due to leaks.
Once contaminants are in the groundwater, the main form of remediation is to pump out the contaminated water and treat it at the surface. This can be a slow process, and preventing the contaminant from travelling significantly during this process can be accomplished by manipulating local groundwater flow through the extraction or injection of water at certain locations. Consider this in the exercise below.
Figure 14.4.11 shows a groundwater contaminant plume in red. The source of the contamination has been removed but if the plume is not dealt with, it will eventually enter the stream and threaten the health of wildlife. Pumping the contaminant from well B for treatment will not be sufficient to prevent some of the contamination from making it to the stream.
What could you do at wells A and C to prevent this? Explain and use the diagram below to illustrate the expected changes to the water table and the movement of the plume.
See Appendix 3 for Exercise 14.6 answers.
Image Descriptions
Media Attributions
- Figure 14.4.1: Wikimedia Commons
- Figure 14.4.2: Colorado Geological Survey
- Figure 14.4.3: “Distribution of Arsenic” by the British Geological Survey (BGS) and the Department of Public Health Engineering (DPHE). Reproduced with the permission of the British Geological Survey © UKRI. All rights reserved.
- Figures 14.4.4, 14.4.5: © Steven Earle. CC BY.
- Figure 14.4.6: “Trail Smelter in Year 1929” by Hughes Brothers. Public domain.
- Figures 14.4.7, 14.4.8, 14.4.9, 14.4.10, 14.4.11: © Steven Earle. CC BY.
- “Adsorb” (with a “d”) is not the same as “absorb” (with a “b”). Water can be absorbed by a sponge. Ions dissolved in water can be adsorbed onto—or desorbed from—the surfaces of clay minerals. ↵

